Electromotive Force
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
Arrhythmia
A condition occurring when the electrical signals that regulate the heart’s rhythm malfunction; resulting in heart beating too fast or irregularly
Implantable Cardioverter Defibrillator (ICD)
Surgically provided to people with the life threatening arrhythmia
Esther Sans Takeuchi
American Chemical engineer to come up with ICDs with increased longevities
Lithium-silver vanadium oxide batteries
What increased the longevity and power output of the ICDs
Alessandro Volta
Italian physicist who coined the term ‘electromotive force’
Voltaic pile
The very first invented battery
one terminal at a higher electric potential than the other terminal, acting as a source of current in a circuit.
A source of emf maintains
there is no net flow of charge within the emf
When the emf source is not connected to the lamp then…
electrons must be moved from the positive terminal to the negative terminal
In order for the emf source to maintain the potential difference between the two terminals….
charge pump
The emf source acts as ___________
Increases the potential energy of the charges and, therefore, the electrical potential of the charges.
What does the emf source moving the negative charges from the positive terminal to negative terminal to maintain the potential difference do?
Work, energy, chemical reactions
____ must be done on the negative charges for them to be moved to the negative terminal. This requires ______, which comes from __________________ in the battery.
ε = dW/dq
Formula emf when there is no current flowing
1 V = 1J/C
Unit for emf and its derivation
Terminal voltage of a battery
Voltage measured across the terminals of the battery.
Ideal battery
An emf source that maintains a constant terminal voltage, independent of the current between the two terminals.
Has no internal resistance and its terminal voltage is equal to the emf of the battery
chemical
The combination of _________ and the makeup of the terminals in a battery determine its emf.
Lead acid battery
The battery used in cars and other vehicles
Anode
(1)
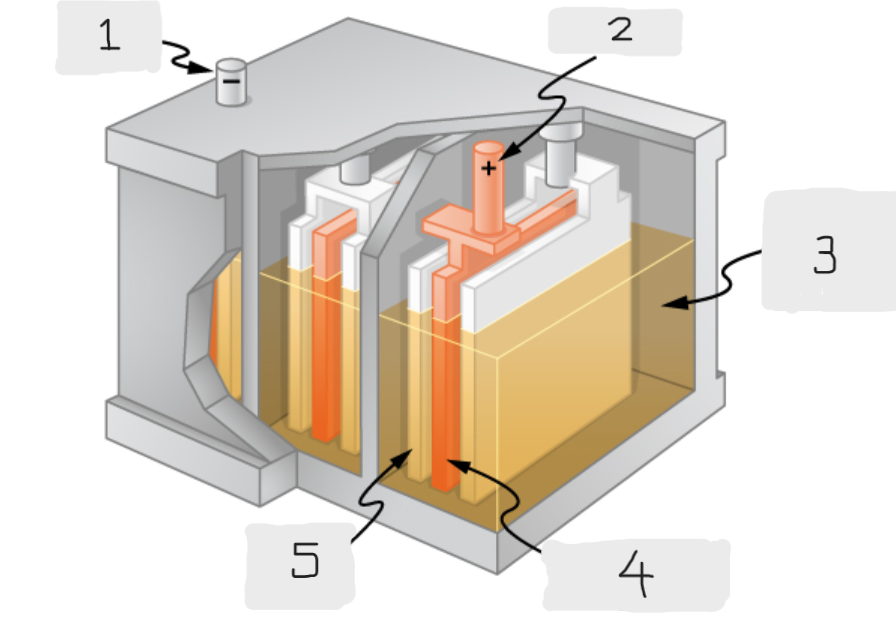
Cathode
(2)
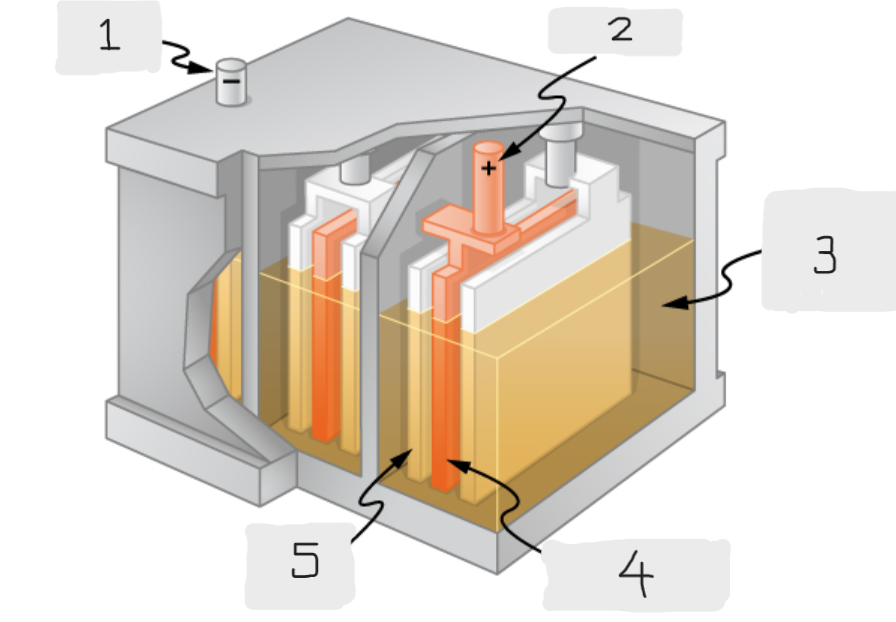
Sulfuric acid
(3)
Also, is considered as the electrolyte of the system
Both the plates immersed in this chemical
This chemical conducts the charge as well as participates in the chemical reaction
Lead Oxide
(4)
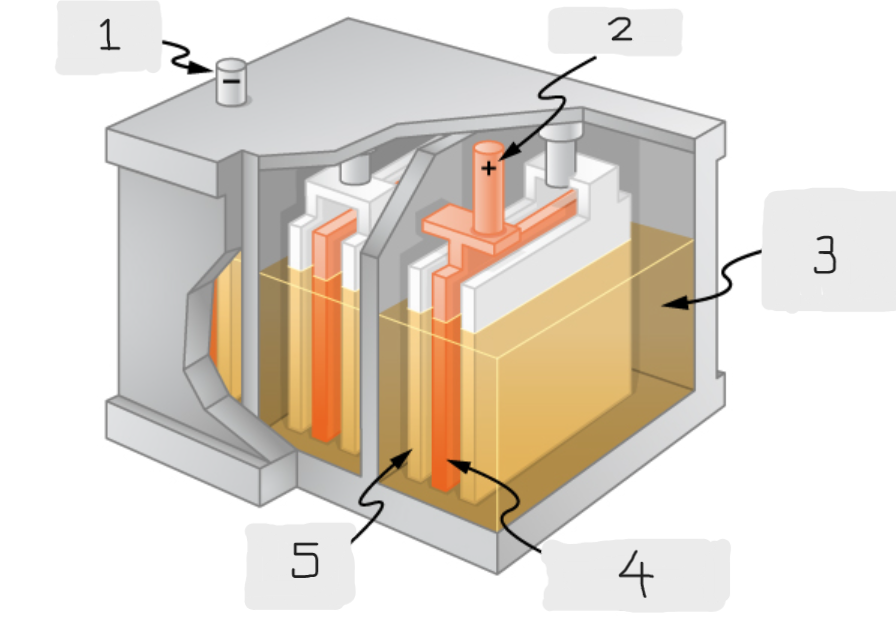
Lead
(5)
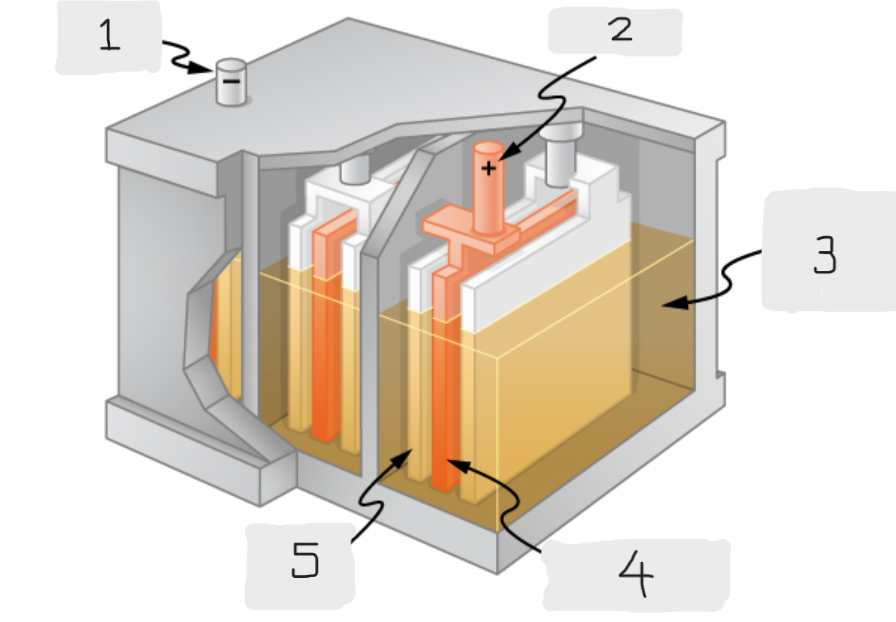
Positive terminal
The terminal of the cell inside the lead acid battery that is connected to the lead oxide plate
Negative terminal
The terminal of the cell inside the lead acid battery that is connected to the lead plate
anode, cathode, chemical reactions, closed-circuit, supply, cathode
In the lead acid battery, two electrons are forced onto the _____ of the cell and two electrons are removed from the _______ of the cell.
This is done by the _________________. It requires the circuit to be a ______________ because this will ______ the electrons to the _______ in the first place.
Lithium-ion batteries
Other types of batteries that are found in many devices.
Function in the same general way as lead-acid batteries but utilize different chemicals
graphite, silicon-based material, metallic lithium
In many cases anode is composed of ________, ______________________, or ________________.
Lithium oxide compound, manganese, cobalt, lithium salt.
In many cases cathode is often composed of ________, which may also contain _________, __________, or other materials.
And the electrolyte is usually a type of ___________.
Internal resistance
The amount of resistance to the flow of current within the voltage source.
Depleted battery
Refers to a battery that has lost its charge and no longer can be used.
depleted
Internal resistance increases as a battery is ________
Oxidation of the plates or the reduction of the acidity of the electrolyte
Two reasons that can make the battery depleted.
Magnitude and direction of the current in the battery, its temperature, and its history.
Three things on which the internal resistance may depend on.
Rechargeable nickel-cadmium cells
It’s internal resistance depends on how many times and how deeply they have been depleted
Idealized emf source and an internal resistance
Two things that is consisted inside the simple model of a battery.
V(terminal) = ε - Ir
Formula for the terminal voltage of the battery.
decreases, increases
The terminal voltage _________ as the current _________ due to the potential drop I*r of the internal resistance.
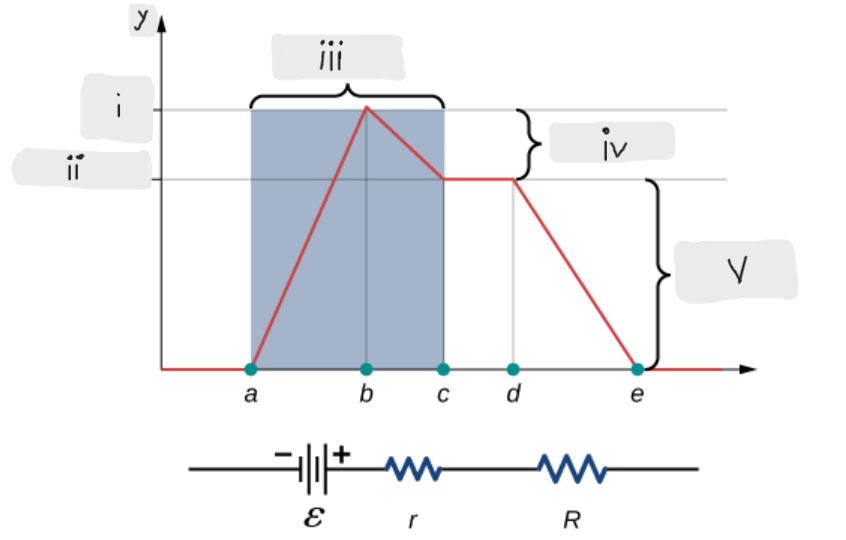
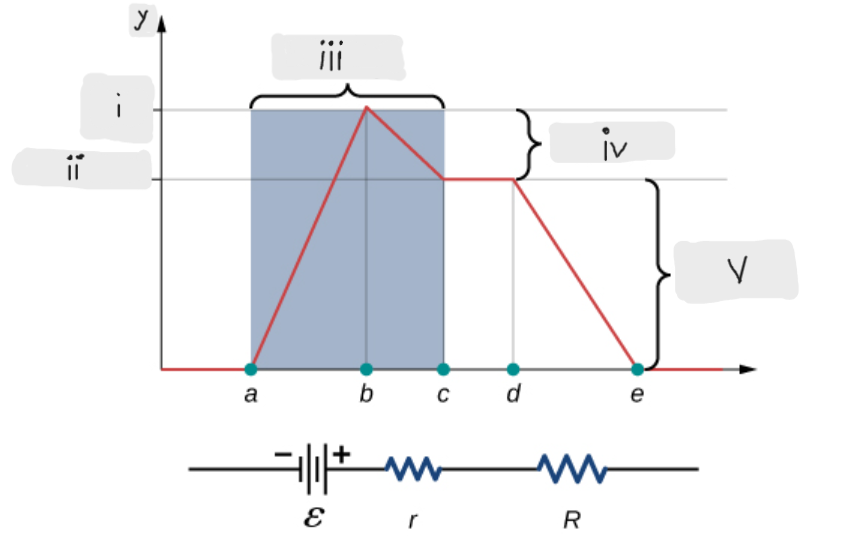
Volts
(y)
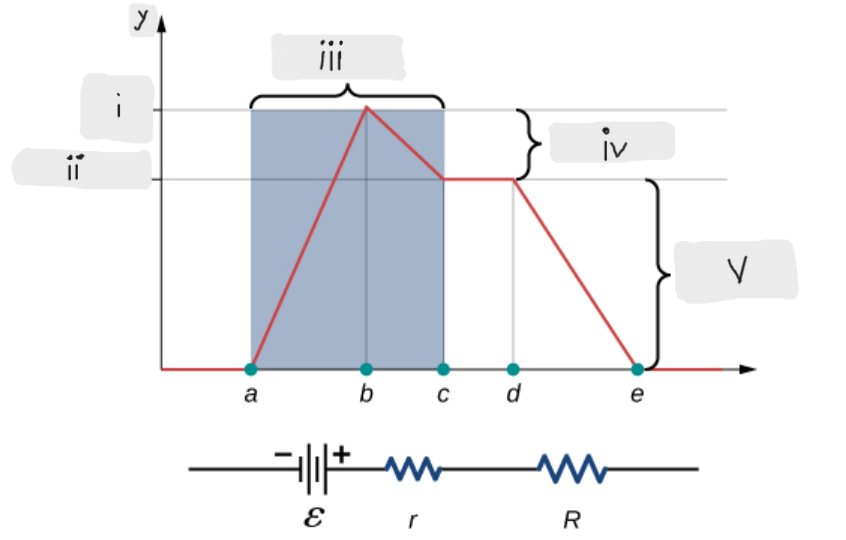
Battery
(iii)
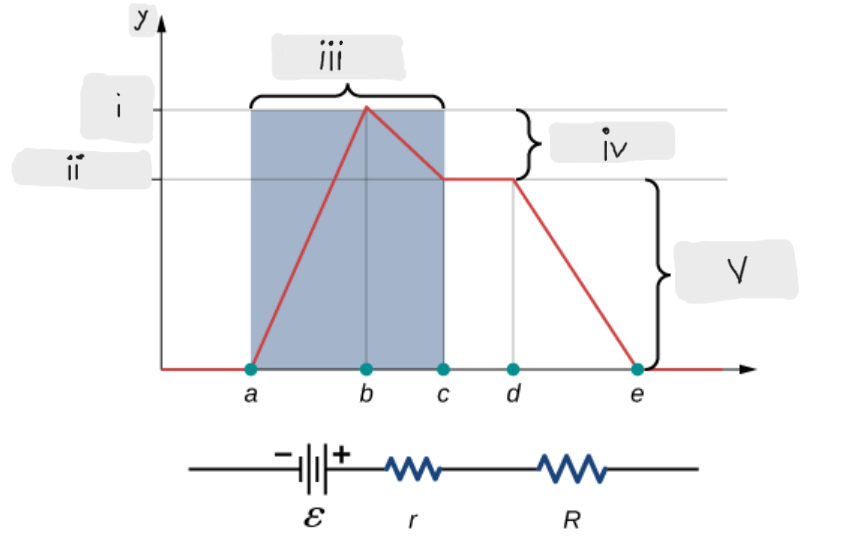
ΔV = I*r [The potential drop across the internal resistor]
(iv)
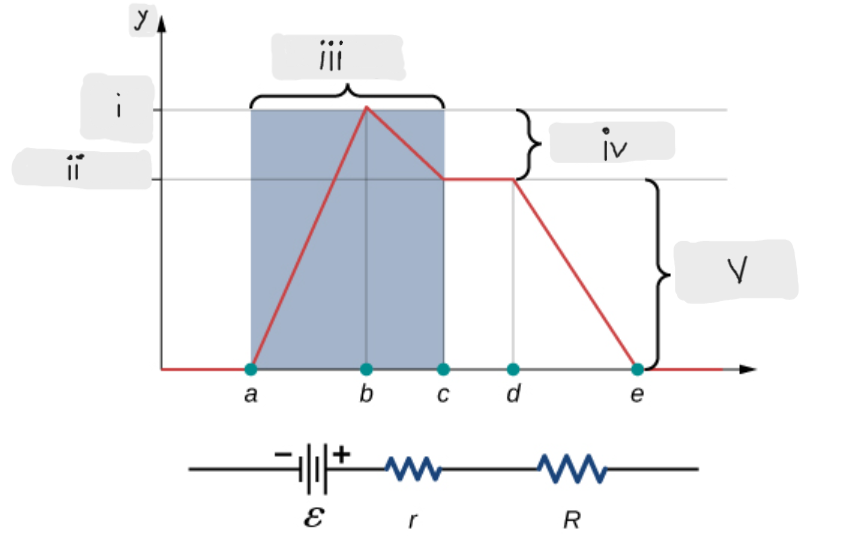
ε - I*r [That’s what the terminal voltage of the battery is]
(ii)
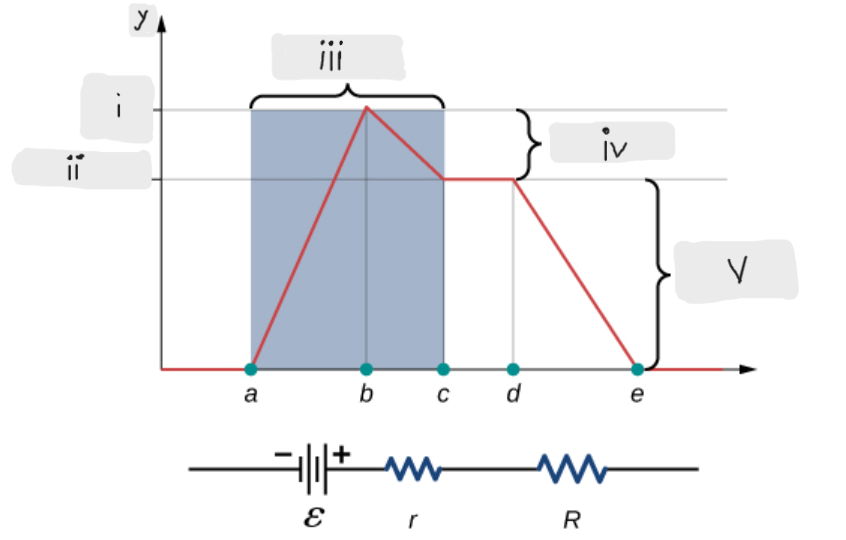
ΔV = I*R = ε - I*r [Potential drop across the load resistor, which is equal to the terminal voltage across the battery]
(v)
I = ε/(r + R)
Formula to find the current through the load resistor
Indicates that smaller the internal resistance, the greater will be the current supplied by the voltage source to its load resistor.
ε [Done by the chemical reactions doing the work on the charges]
(i)
Yes, it is true
Is it true that the internal resistance of a rechargeable battery increases as the number of times the battery is recharged increases.
Its terminal voltage will decrease and the battery may overheat due to the increased power dissipated by the internal resistance.
The two effects that the increased resistance may have on the battery.
Battery testers
A device that measures the terminal voltage under a load to determine condition of a battery.
Provides a measurement of the internal resistance of the battery
Uses small load resistors to intentionally draw current to determine whether the terminal potential drops below an acceptable level.
weak, low terminal voltage
If the internal resistance of the battery is high, then the battery is ____, as evidenced by its ____________________.
Battery charger
Devices that reverses the normal direction of the current through a battery, reversing its chemical reaction and replenishing its chemical potential.
To do all this its voltage output must be greater than the emf of the battery. Causing the terminal voltage of the battery to be greater than its emf, as I is now negative
Used routinely in cars and in batteries for small electrical appliances and electronic devices.