Ch 89-90 + F Ch 18
1/134
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
135 Terms
Developmental Orthopedic Disease (DOD)
Encompasses virtually all noninfectious orthopedic deranged developments of growing young horses Osteochondrosis is the most common Also includes cervical vertebral stenoic myopathy (Wobbler disease), collapse of cuboidal bones, angular and flexural limb deformities, subchondral bone cysts, and physitis
Osteochondrosis (OC)
Disorder itself
Osteochondritis
Inflammatory response to the disorder
Osteochondritis Dissecans (OCD)
An area of cartilaginous or osteochondral separation is present
OC latens
Focal osteonecrosis of the resting zone of the growth cartilage with adjacent vascular necrosis
OC manifesta
A later stage that includes focally impaired osteochondral ossification and cartilage retention
OC Dissecans
Characterized by cleft formation through the necrotic cartilage
Juvenile Osteochondral Conditions (JOCC)
Developmental orthopedic disorders that are related to the immature joint or growth plate Includes OCD, cuboidal bone disease, and various other forms of failure of the immature skeleton, but not cervical vertebral stenotic myopathy, flexural limb deformities, or angular limb deformities
Endochondral Ossification
In all mammals, the primordial skeleton is laid down first as a cartilaginous structures that starts to transform into bone during the fetal stage, a process that continues until well after birth
Fetal cartilaginous structures are well vascularized by vessels running through so-called cartilage channels
Ossification of the primary centers of ossification in the diaphyses of the long bones starts early in fetal life, and at the time of birth, all diaphyses are bony structures
Many secondary centers of ossification located in the epiphyses of the long bones and in other sites such as apophyses and cuboidal bones, are still partly cartilaginous at the time of birth
Longitudinal Growth of Long Bones
Longitudinal growth of long bones occurs at the growth plates or physes Chondrocytes originating from a germinal layer of cells (resting cells) undergo mitosis, proliferate, and subsequently hypertrophy and undergo apoptosis The chondrocytes are encased by a scaffold of extracellular matrix which forms the basis for the apposition of primary bone by osteoblasts that originate from the metaphysis The primary spongiosa that is formed will undergo continuous remodeling under the influence of biomechanical loading according to Wolff's law during the entire growth period of the foal Process of cartilage remodeling, followed by calcification of cartilage, deposition of primary bone, and successive remodeling into bony trabeculae as occurs in the young, growing animal, is known as endochondral ossification
Growth Process in the Epiphysis of Long Bones
In the epiphyses of the long bones, a similar growth process takes place, but is less advanced than in the diaphyses at birth Initially, there is a complete ring of cartilage around the ossification center that is located in the center of the epiphysis, connecting the cartilage at the articular side with the growth plate Ossification of this cartilage ring takes place first at the border of the physis and at the perimeter of the epiphysis The thick cartilage mass at the articular side of the epiphysis functions as a type of growth plate with simultaneously occurring processes of growth, remodeling, and ossification It is at this level that the characteristic lesions of equine OC develop After cessation of growth, a considerably thinner layer of articular cartilage remains in the mature animal Although macroscopically very similar, this layer is right from the start distinct from the growth cartilage
Joints Most Frequently Affected by OC
OC is most common in tarsocrural, femoropatellar, and metacarpo/metatarsophalangeal joints, but has been described in most other diarthrodial joints as well
Breed Differences in the Prevalence of OC Lesion Locations
In Warmbloods and Standardbreds, tarsocrural OC is most frequent, whereas in racing Thoroughbreds femoropatellar OC is predominant
Unillateral vs Bilateral OC Lesions
Most lesions present unilaterally, but are often found to be bilateral in the tarsocrural and femoropatellar joints and bilateral or even quadrilateral in the MCP/MTP joints
Concomitant Occurrence of OC Lesions in Other Joints
Concomitant occurrence in other joints or joint pairs is much less common, possibly because of the differences in time windows during which OC lesions develop in different joints
OC Predilection Sites in the Tarsocrural Joint
Cranial end of the distal intermediate ridge of the tibia
Distal end of the lateral trochlea of the talus
Medial malleolus of the distal tibia
OC Predilection Sites in the Femoropatellar Joint
Lateral trochlear ridge of the femur
OC Predilection Sites in the Shoulder Joint
Glenoid and the humeral head
OC Predilection Sites in the MCP/MTP Joints
Dorsal aspect of the sagittal ridge of MCIII and MTIII
Difference in OC Joint Fluid vs Traumatic Joint Fluid
OC joint fluid has in increase of the collagen degradation marker C2C while traumatic joint injury fluid has an increase in the collagen synthesis marker CPII
Timeline of Appearance and Regression of OC Lesions in the Tarsocrural Joint
Lesions originating during the first few months of life had mostly resolved at 5 months of age
Remaining lesions did not resolve
Timeline of Appearance and Regression of OC Lesions in the Femorpatellar Joint
Lesions originated from approximately 3 months onward, peaked at 6 months, and reached a stable condition around 8 months of age with most lesions healed
What is the age at which no further OC lesions will form nor will existing large lesions resolve?
At 12 months, for all joints, no major OC lesions should be formed, nor will existing large lesions be expected to resolve Resolution of minor lesions has been reported up to 24 months of age
Cartilage Turnover in Mature vs Young Growing Horses
Collagen turnover in mature cartilage is known to be virtually nil, but in young growing individuals, continuous remodeling and formation of cartilage takes place Huge difference in metabolism is reflected by the presence of synovial fluid levels of certain proteinases MMP-3, stromelysin levels are increased 80-fold in fetal joints compared to joints from mature individuals At 5-11 months of age, MMP-3 levels decrease dramatically, but are still two-to threefold higher than in mature joints
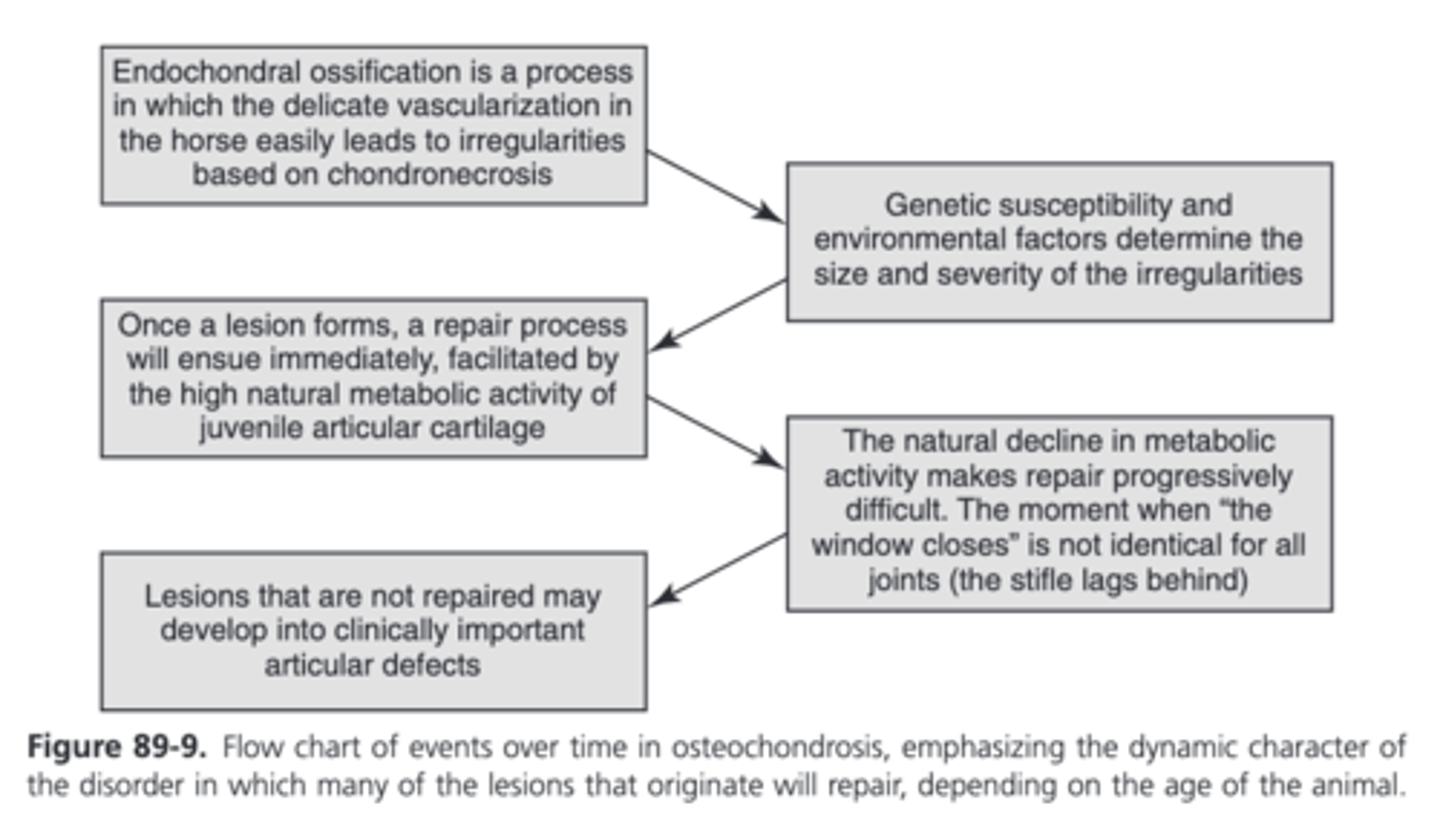
What are the two processes that clinical OC can be considered as the final outcome of?
The initiation of lesions by specific etiologic factors
Ensuing repair process
Flow Chart of Events Over time in Osteochondrosis
Vascular Events in Early OC: the Early Pathogenetic Mechanism
The thick layer of epiphyseal cartilage of the growing joint that is destined to change into bone via the process of endochondral ossification is nourished by vessels running through so-called cartilage canals
With ongoing ossification, these canals are obliterated in a process called chondrification, the timing of which is joint dependent Patent cartilage canals are not seen in proximal phalanx after 3 weeks of age, whereas they are still present at 4.5 months in the femoral condyle (but disappear by 7 months of age) The timing of canal abolishment in distal tibias was in between
The early pathogenetic mechanism of OC was the formation of chondronecrotic areas, caused by damage to cartilage canals, especially to the anastomosing branches that run through the ossification front from the bone marrow These initial chondronecrotic areas were clinically silent, not visible macroscopically or with the help of standard imaging methods and were called OC latens
Depending on environmental factors and the efficacy of the repair processes, these lesions would resolve or become clinically apparent, in which case they are designated as OC manifesta
In a genome-wide association study in pigs, a single nucleotide polymorphism (SNP) in the gene encoding T-box transcription factor 5 (TBX5) (a transcription factor interacting with two genes involved in vascularization) was significantly associated with OC lesion scores
The advancing ossification front induces a change in the arterial supply of the cartilage canals Initially supplied by arteries from perichondral origin, the advancing ossification front engulfs the midportion of the vessels in the canal, necessitating a shift towards subchondral, rather than perichondral arterial sources This shift suggests that crossing the ossification front leaves the vessels more vulnerable to mechanical influences
The secondary repair process follows almost immediately after the formation of the lesion
In one study at the age of 13-15 days, two vessels supplying the epiphyseal growth cartilage of the lateral trochlear ridge of the femur were transected in 10 pony foals which resulted in ischemic chondronecrosis that was associated with a focal delay in endochondral ossification (OC) in foals examined 21 days or more after transection In one foal a pathological cartilage fracture (OCD) was observed 42 days after transection
Vascular pathogenetic mechanism of OC can explain a number of commonly observed features of the disease, such as the joint-specific windows in time (related to joint-specific patterns in the progress of the ossificiation front and subsequent vascular rearrangements) and the frequent bilateral occurrence
The complex vascular rearrangements during the process of endochondral ossification are common to all individuals and not specific for those developing osteochondrotic lesions and offer no explanation for the individual susceptibility for OC It has been suggested that bacterial infections at early age may produce direct damage to the vascular structures, of which a large proportion has been shown to be surrounded by an acellular wall consisting of collagen type I, permitting bacterial binding A variety of more indirectly acting molecular mechanisms related to dysfunction of chondrocytes, extracellular matrix components, and signaling pathways have been suggested to play a role as well
Molecular Events in OC: The Possible Underlying Pathways
Osteochondrotic fragments could not be discerned from surgically created osteochondrotic fragments, however, the OC tissue bed stained positive for chondroitin sulfate and collagen type II, and the fracture bed did not
A comprehensive approach using both proteomics and metabolomics showed involvement of proteins related to cell cycle, energy production, cell signaling and adhesion, as well as chondrocyte maturation, extracellular matrix, and mineral metabolism, the lateral processes signifying a role for the subchondral bone as well
The role of the chondrocyte in OC Failure to undergo hypertrophy has been suggested as a main cause for OC or "dyschondroplasia, however this hypothesis is unlikely Chondrocytes harvested from early osteochondrotic lesions have a higher metabolic rate, but cannot be further stimulated to a higher level than chondrocytes from normal cartilage When harvested from longer-existing lesions, their metabolic rate is lower than in normal cells and stimulation is not possible This phenomenon may indicate a reactive upscaling of metabolic activity in response to lesion formation, which, when repair is unsuccessful, may develop into exhaustion and loss of vitality of the chondrocytes
Matrix Components in Osteochondrosis
Distribution of collagen VI was different in OC lesions compared to normal tissue
Differences in posttranslational modifications of collagen type II have been demonstrated in samples from early lesions
There was strong TGF-B mRNA expression in chondrocyte clusters immediately surrounding an OC lesion
OC cartilage showed increased expression of collagen types I, II, III, and X, and of MMP-13, ADAMTS-4, and TIMP-1 and decreased expression of TIMP-2 and TIMP-3
Expression of MMP-16 or membrane-type matrix metalloproteinase-3 (MT3-MMP) is not significantly altered in osteochondrotic cartilage
A strong increase in cathepsin B activity in chrondrocyte clonal clusters in OC has been demonstrated
Pellet cultures produced from OC tissues contained significantly less GAGs and there was an increase in activity of gelatinases (MMP-2 and MMP-9) in osteochondrotic cartilage
IGF-1 was found to be upregulated in osteochondrotic tissue, but was judged to be most likely related to the repair response rather than being primary
Signaling Pathways in Osteochondrosis
Significantly decreased Wnt-11 and increased B-catenin, Wnt-5b, Dkk-1, Lrp6, Wif-1, Axin1, and SC-PEP gene expression could be shown in early OC cartilage canal chondrocytes compared to controls
A Wnt signaling inhibitor, sclerostin, was strongly upregulated in OC lesions
Wnt signaling is known to regulate mitochondrial physiology and insulin sensitivity and abnormal mitochondria have been observed in the deep zone of OC cartilage
Heritability
Heritability (h2) is a statistic that estimates how much variation in a phenotypic trait in a population is a result of genetic variation
Can vary between 0 and 1.0
The higher the value, the bigger the influence of selective breeding on that trait
Heritability of OC in the Tarsocrural Joint
In most breeds heritability is highest for the tarsocrural joint with an average value around 0.30 but covering a range from 0.04 (Coldbloods) to 0.52 (Standardbred trotters)
Heritability of OC in the MCPJ/MTPJ
Approximately 0.15
Heritability of OC in the Stifle
In the stifle these values are lower, not rising above 0.10 with values as low as 0.05 for OC and 0.02 for OCD in the Dutch Warmblood
Heritability and Selecting for/against OC
Heritability measures the proportion of the phenotypic variance that is the result of genetic factors In theory, the higher the heritability, the quicker progress to eliminate a defect can be made by selection In practice, selection against OC has been proven extremely difficult, and progress, if any, has been slow Explanations OC is heavily polygenic with different genes involved in different joints resulting in a different h2 per joint Indications that different manifestations of OC (fragmentation versus flattening) may represent different traits Dynamic nature of OC Some traits that are known to be implicated in OC (such as a high growth rate) are seen as desirable and actively selected for or that certain conformational traits are both related with good athletic performance and increased risk for OC
Molecular Genetics of OC
Linkage and association analyses and genome-wide association studies have identified regions of the genome associated with some phenotypic manifestation of OC on about 2/3 of the 33 chromosomes of the horse
There is little overlap between MCP/MTP OC and tarsal OC, suggesting a different genetic background
Many of the identified quantitative trait loci (QTL) are breed specific
Many loci positively associated with desired traits in breeding have a relation with OC too
A different approach using leukocytes as cell source for DNA instead of articular tissues, identified dysregulation of a number of pathways, among others Wnt, Ihh, and TGF-B signaling in OC affected animals
The gene mannosyl glycoprotein acetylglucosaminyltransferase (MGAT4A) was the only gene to be strongly upregulated in all age groups with OC This gene is implicated in the intracellular transport of glucose and it can be conjectured that expression of this gene may be triggered by high levels of glucose and triglycerides, as resulting from the intake of high-energy diets Transient hyperglycemia peaks may lead to long-lasting changes through epigenetic changes, including superoxide anion-mediated mitochondrial damage Abnormal mitochondria and endoplasmic reticulum have been observed in the deep zone of OC cartilage
Environmental factors contribute relatively more to the manifestation of OC than genetics
What are the two major environmental influences playing a role in equine OC?
Loading
- Mainly determined by the exercise regimen
- Roughness of the terrain and conformation also may be contributing factors
Nutritional factors
- Can affect growth rate but may also influence hormonal balances, especially with respect to glucose metabolism, or can affect the mineral and trace element status of animals
Environmental Factors Affecting OC - Loading
A triggering role for biomechanical forces fits very well with the early pathogenesis of vessels in cartilage canals passing through a time window of enhanced vulnerability Whether or not a lesion will develop will be determined by the character of the biomechanical insult (magnitude, direction or force, repetition) and the degree to which the resistance of the vessel is impaired
Biomechanical (over)loading of joints may be caused by a variety of factors Irregular access to pasture Keeping animals in very large plots "mixed housing" (stabling overnight and pasture access during the daytime as opposed to the same environment day and night) Rough and slippery grazing grounds
Environmental Factors Affecting OC - Nutrition - Energy Intake
Growth rate is associated with OC, but this is irrespective of whether the high growth rate is caused by high nutritional levels or linked to genetic factors
Not so much overall growth rate but growth at defined age intervals that becomes significant
A high growth rate based on nutritional level is almost invariably linked to excessive intake of carbohydrates, often in an easily digestible form, such as concentrates This nutrition leads to a strong postprandial hyperinsulinemia This hormonal response varies between horses and may explain some of the variation in OC susceptibility Horses with OC have been shown to have higher post-prandial glucose and insulin responses to feeding higher-grain ratios than did unaffected horses
Insulin and its derivatives IGF-1 and IGF-II have a direct effect on the process of endochondral ossification, acting as mitogens for chondrocytes and stimulating chondrocyte survival or suppressing apoptosis OC-affected foals have a significantly lower IGF-I activity than OC-negative foals
Insulin also stimulates a rapid removal of the thyroid hormones T3 and T4 from the circulation T3 and T4 are involved in the final stages of chondrocyte differentiation and in the invasion of growth cartilage by blood vessels prior to its conversion to bone The effect of carbohydrates on thyroid hormone levels can be demonstrated in weanlings, but not in yearlings
Nutrition-related hormonal imbalances play a role in the development of equine OC, probably in increasing vulnerability for early vascular damage, however it is highly unlikely that it is the sole etiologic factor, as OC lesions can also be found in horses eating normal diets without any abnormalities in their insulin metabolism
Many lesions provoked by the administration of high-carbohydrate diets were similar, but not identical to clinical OC lesions
Many experimentally induced lesions were seen in the growth plate, where clinical OC is rarely, if even seen in horses
Environmental Factors Affecting OC - Nutrition - Imbalances of Minerals and Trace Elements
Imbalances in trace elements, in particular copper and its antagonists zinc and cadmium, have been implicated in the development of OC Mechanism was thought to act via lysyl oxidase, a copper-dependent enzyme that is essential for the formation of collagen cross-links Copper is no longer incriminated as being the main culprit for the development of OC
The calcium/phosphorous ratio is important for bone metabolism and (severe) aberrations will cause various bone disorders High calcium levels were shown to have no influence on the incidence of OC in foals, but high levels (four times the NRC recommendation) of phosphorous resulted in significantly more lesions Mechanism of action was suggested to be the induction of secondary hyperparathyroidism, which would lead to increased osteoporosis and subsequent weakening of the subchondral bone
Conservative Treatment of OCD
Rest and controlled exercise
Systemic NSAIDs and intraarticular medication may be administered but are not seen of great value
Can only be successful in either very young animals in which there is still good capacity for regeneration, or in very mild cases
Stifle lesions change over the longest period of time
When is healing of OCDs with conservative treatment deemed likely?
Healing with conservative treatment is deemed likely when lesions are less than 2 cm long and less than 5 mm deep without radiographic fragmentation
When is conservative treatment advised with OCDs of MCIII/MTIII?
When flattening without fragmentation (type IOC) occurs in the sagittal ridge of the MCIII or MTIII initial conservative treatment is advised
Conservative Treatment of OC in the Scapulohumeral Joint
Conservative treatment of OC in the scapulohumeral joint was earlier seen as having a very poor prognosis, recent data suggests it may be a worthwhile option in mild cases where the glenoid cavity is involved
Prognosis for OCDs in the Femoropatellar Joint
64% success rate reported for the femoropatellar joint in a mixed population of racehorses and nonracehorses
Horses with smaller lesions (Grade I, <2 cm in length) were more successful than horses with larger lesions (Grade II, 2-4 cm and Grade III, >4 cm)
65% complete functional recovery in a more recent study in which depth of lesion was significantly associated with short-term complications (effusion and lameness), but not with the long-term outcome (UpRichard et al, 2013)
Involvement of structures other than the lateral trochlear ridge (patella, medial trochlear ridge) was associated with a worse prognosis
In another study, 76% of arthroscopically treated horses were able to perform as intended (Vatistas, Wright, and Dyson, 1995)
Prognosis for OC Lesions in the Tarsus
A recent study in a Standardbred trotters and pacers showed that when undergoing early removal of tarsal OC lesions, affected horses can be expected to perform equivalently to their unaffected counterparts (McCoy, Ralston, McCue, 2015)
In a large survey of horses operated for tarsocrural OC, success rates were 73% in racehorses and 83% in nonracehorses (McIlwraith, Foerner, and Davis, 1991)
Synovial effusion resolved in 89% of racehorses and 74% of nonracehorses
Score reductions for lameness and reaction to the flexion test of 80% to 90% and resolution of joint effusion in around 50% of cases of tarsocrural OC have been reported in a mixed population of Standardbreds and Warmbloods
Prognosis for OCDs of the MCP and MTP Joints
In the MCP and MTP joints, a discrimination is made between lesions type I (flattening only), type II (flattening with fragmentation), and type III (flattening with or without fragmentation at the lesion site and a loose body present) of the sagittal ridge of MCIII/MTIII
Type I - treat conservatively
Type II and III - treat surgically shortly after diagnosing as treatment at a later stage is associated with the development of osteoarthritis
90% return to athletic activity reported if the lesion is located in the more proximal part of the sagittal ridge, but lower rate for lesions in the weight-bearing area
Prognosis for OC of the Shoulder
Shoulder OC has the least favorable prognosis
One study reports a favorable outcome of only 15% in racehorses (but better in nonracehorses) suffering from shoulder OC, which was similar after surgical or conservative treatment (Jenner et al, 2008)
Other reports mention successful outcome in approximately 50% of cases (McIlwraith, 2013)
Effects of OC on Performance
For tarsocrural OC in Standardbreds, general trend is that fewer and smaller lesions may delay the start of the racing career but will have very little, if any, effect on performance
Severe or multiple abnormalities significantly compromise a potential future racing career
Racing performance in Thoroughbreds treated for OC in the femoropatellar joint was not different from that in unaffected siblings, but fewer horses raced as 2 year olds and earnings were less, both at 2 and 3 years of age
In a more recent study in racing Thoroughbreds (flat and hurdle racing), fewer horses with radiographic findings raced as 2 year olds and fewer were placed as 3 year olds compared to horses without radiographic anomalies (Verwilghen et al, 2013)
In show jumpers, no differences between horses with tarsocrural OC and controls could be established, but OC of the femoropatellar and MCP/MTP joints significantly affected performance
Pathogenesis of SCLs
Originally SCL of the equine stifle were described as a manifestation of the osteochondrosis (OC) complex, resulting from retained, thickened, necrotic cartilage in the physis that infolds into the subchondral bone in the weight-bearing areas of the joint
Horses are usually of a young age
Another pathologic mechanism responsible for the development of SCLs is trauma to the articular cartilage, subchondral bone, or both Creates communication between the subchondral bone and the joint, allowing synovial fluid to gain access to the subchondral region under pressure and inducing necrosis of the adjacent bone which contributes to cyst formation
An articular fissure or fracture can lead to SCL formation In almost 1/3 of the cystic lesions, a concomitant fissure was visible on CT Most of the fissure lines had a midsagittal course and occurred in the proximal aspect of P1
Trauma can cause subchondral bone damage and bone ischemia and necrosis, followed by revascularization and resorption of necrotic bone, leaving a subchondral lesion
Bone trauma and secondary subchondral necrosis can also be the result of areas of contact of damaged articular cartilage affected by SCL
SCLs have been recognized after articular sepsis
Inflammation has been suggested as a contributing factor Analysis of the tissue lining and fluid found in SCLs in horses suggests that upregulation of inflammatory mediators may contribute to bone resorption and impaired healing of these lesions
Most Common Location of SCLs
Medial condyle of the femur
Percentage of SCLs in the Medial Femoral Condyle
45.8%
Percentage of SCLs in the Phalanges
26.2%
18.5% of all phalanges involved the distal sesamoid bone
Percentage of SCLs in the Carpal Bones
7.1%
Percentage of SCLs in the Third Metacarpal and Metatarsal Bones
6%
Macroscopic and Histologic Findings in SCLs
Show marked variation in size (from several millimeters up to >3 cm) and shape; may be shallow or deep (>10 mm) and may be dome-shaped, spherical, flattened, or without a particular shape
May be mild-to-intense sclerosis surrounding the cyst depending on the stage of development
Most lesions communicate with the articular cartilage
In histologic sections, most SCL are located within epiphyseal trabecular bone, rather than the subchondral bone plate
SCLs have a fibrous lining and may contain some fibrous tissue and/or gelatinous material Some lesions also contain fibrocartilage and necrotic bone
Cystic wall consists of elongated fibroblasts aligned parallel to collagen bundles, scattered macrophages, and polymorphonuclear cells
Hypervascularity and thickened bone trabeculae can be noticed in the adjacent bone
Cartilage overlying an SCL is generally normal looking except close to the canal, where signs of matrix degradation can be detected histologically on surgical explants
Clinical Symptoms of SCLs
May or may not be associated with lameness
Lameness is attributed to pain from the joint (synovitis), the subchondral bone (increase in intraosseous pressure), or an increase in intracystic pressure, or a combination
In SCLs caused by osteochondrosis, lameness commonly develops at the start of training
In middle-aged to older horses, a traumatic event or correlation to ongoing intraarticular inflammation may be associated with onset of lameness
Diagnosis of SCLs - Radiography
Typical radiographic finding is a dome-shaped or round-to-oval subchondral lucency with a variable surrounding sclerotic rim
DP views best suited for detection of cystic lesions in the distal limb
SCLs of the MFC usually diagnosed by a craniocaudal image but may be seen more readily in a caudolateral-craniomedial oblique or flexed lateral image
Joint in the contralateral limb should also be evaluated because these lesions frequently occur bilaterally
In a recent study, only 79% of SCLs diagnosed on CT were identified radiographically (Schon et al, 2017)
Diagnosis of SCLs - Computed Tomography
CT is more accurate in the detection of small SCLs, their exact localization within the sagittal plane, as well as the detection of concomitant fissure lines
Fissure lines often associated with SCLs of the proximal P1, either mid-sagittal or parasagittal
CT also allows detection of communication between the SCL and adjacent articular cartilage
In one study this was evident in 40/42 SCLs (Schon et al, 2017)
Commonly missed radiographically but appears to be a common characteristic of SCLs
Recent study used CT to determine the morphologic characteristics of SCLs of the MFC (Walker et al, 2016)
In the sagittal plane all the lesions showed an articular communication in the cranial 15-20% of the MFC
Small and intermediate-volume SCLs were irregular and multilobulated, whereas large-volume SCLs had a more spherical appearance
Nonsurgical Treatment of SCLs
Rest, NSAIDs, vitamin supplements, anabolic drugs
Not successful, one study reported failure rate of 66% (von Rechenberg and McIlwraith, 1998)
Other reports listed success rates of 45-64%
In one study, no significant differences were found in racing results between yearlings with radiographically diagnosed SCLs in the MFC and unaffected horses, leading to the prediction that some lesions spontaneously resolve without treatment (Whitman et al, 2006)
Limited exercise and intraarticular medication has a reported success rate of 64% with horses less than 3 years of age having a better prognosis for soundness
Benzyopyrone for Treatment of SCLs
Benzyopyrone has been used systemically in horses with SCLs with 12/19 returning to normal use
Assuming pain associated with these lesions originates from increased intraosseous pressure, this drug should decrease the osmotic pressure in the bone that leads to lameness
Corticosteroid Injection for Treatment of SCLs
Intraarticular injection of steroids (and hyaluronan) is often the first approach in the treatment of SCLs and leads to immediate improvement of the lameness, however risk of recurrence of clinical signs is very high
Injecting corticosteroids into the lining of the SCL under arthroscopic or ultrasonographic guidance is based on work where inflammatory mediators were detected in the cystic contents leading to bone resorption
Corticosteroid is deposited throughout the cyst lining, with the most effective technique involving multiple redirections of the needle to distribute the medication
In one study, lameness resolved in 67% of horses receiving injections and 77% were classified as successful based on follow-up lameness examination (Wallis et al, 2008)
Arthroscopic Approach for Surgical Treatment of SCLs
Once the cyst has been identified, a rongeur can be used to remove the articular cartilage overlying the SCL
Once all the cartilage not supported by underlying bone is removed, the contents of the cyst are evacuated with the help of a curette or motorized shaver
Osteostixis of the adjacent bone is not recommended because it can lead to expansion of the cyst
The contents and lining of the cyst are removed until the subchondral bone is visible
Transcortical Approach for Surgical Treatment of SCLs
Most of the SCLs of the distal limb are not accessible through an articular approach and have to be debrided transcortically
Should be performed under digital radiography, fluoroscopy, or CT guidance
Skin incision over selected location advanced down to the bone
Under fluoroscopic control, a 3.5 mm pilot hole is drilled through the bone into the cyst
Once placement of the drill tip into the SCL has been verified, the drill hole is enlarged with a 5.5 mm drill bit to accept a small arthroscopic curette
Cancellous Bone Graft for SCLs
Packing the lesion with autogenous grafts has been recommended but a study comparing healing of surgically created subchondral defects filled with compacted cancellous bone grafts with empty defects revealed no difference in the healing patterns after 6 months. This type of management is no longer used
Mosaic Arthroplasty for SCLs
Autologous osteochondral grafting
One study concluded that material properties of the grafts from the trochlear groove and axial aspect of the lateral trochlear ridge were the closest match for those found in the medial condyle, whereas properties of the lateral condyle were most similar to those found in the trochlear groove and axial aspect of the medial trochlear ridge of MCIII/MTIII (Changoor et al, 2006)
In one study grafts of bovine, ovine, human, and equine origin were implanted in the femoral condyles of sheep (Waselau et al, 2005)
At 6 months equine grafts showed the best score for cartilage surface integrity and highest percentage for bone, thus having a better performance compared with grafts of other species
Performed in a clinical case series of 11 patients where grafts were harvested from the abaxial border of the medial femoral trochlea of the unaffected limb (Bodo et al, 2004)
Grafts implanted through a small arthrotomy or by arthroscopy
All horses improved postoperatively, 10 had successful outcomes with radiographic evidence of successful graft incorporation and 7 returned to a previous or higher activity level
On follow-up arthroscopy there was successful reconstitution of a functional gliding surface with survival of transplanted hyaline cartilage
One horse had delayed incorporation of a graft because of a technical error but became sound
One horse had recurrence after 4 years of work and soundness
Tricalcium Phosphate Granules for SCLs
Tricalcium Phosphate (TCP) granules used as filling material for SCLs of the distal limb following transosseous curettage
When there is a large communication between the cystic lesion and the joint cavity, filling of the SCL with TCP granules is not recommended because of possible spread into the joint
Before implantation, granules are placed in a syringe, autologous whole blood is added, and a vacuum is applied for several minutes to evacuate air from the granules
Drill hole into the cyst and cyst cavity filled with the mixture
Parathyroid Hormone Application for Treatment of SCLs
In humans when administered systemically and intermittently in low concentrations it has a strong anabolic effect on bone
In a clinical case series of 15 horses that were lame because of an SCL at different anatomic locations, 11 became sound after debridement and filling of the lesions with PTH1-34 in a fibrin hydrogel (Jackson et al, 2012)
Cysts curetted under arthroscopic supervision or through a transosseous approach followed by injection of the activated hydrogel
In arthroscopic cases, debridement of the cyst cavity was performed under fluid distension, fluid irrigation was then stopped and joint expansion was maintained by switching to CO2 and the cystic cavity was dried and blood and fluid removed
Gelation of the hydrogel took place within 1-3 minutes
When the transosseous approach was used the lesions were flushed after curettage and as much liquid as possible was removed through suction
Autologous Chondrocyte Implantation for SCLs
Gold standard for repair of large cartilaginous lesions in humans
A recent study demonstrated improved healing in the short (8 weeks) and long term (8 months) following implantation of autologous chondrocytes transduced ex vivo with a self complementary adeno-associated virus overexpressing IGF-1 in the equine femoral trochlea (Ortved at al, 2015)
Bone Morphogenetic Protein-2 for SCLs
In one study the effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) in three horses suffering from five SCLs in the pastern joint was investigated (Jackson et al, 2017)
In all three horses, treatment resulted in increased bone density, decreased cyst size, and an absence of lameness
Screw Insertion for SCLs
In a recent study, 20 horses with lameness attributable to an SCL in the MFC were treated with a transcondylar 4.5 mm cortex screw inserted in lag fashion without debridement of the lesions (Santschi et al, 2015)
By 120 days lameness was eliminated in 15 horses and the SCL area had decreased by 50% or more
Good option for lesions in the distal limb
Often traumatic in origin and in almost 1/3 of the cases a concomitant fissure line is present when evaluated on CT
What are the two rationales for treating SCLs with bone screws?
To improve bone remodeling in the cyst because of changes of the biomechanical environment in SCLs without concurrent fissure lines
To stabilize fissure lines that can be a causative factor for the SCL
Miscellaneous Techniques for Treating SCLs
Autologous patient-side grafting using bone marrow aspirate concentrate and PRP with TCP
Injection of hydraulic biodegradable cement
Titanium spongiosa balls of 5 mm used as filling material after debridement of SCLs
Surface microtopography of the implants has a major effect, influencing the interaction between the implant's surface and its biological environment, stimulating cell attachment, spreading, and proliferation of osteoblastic cells
Purpose is to enhance bone formation in the cystic cavity
In cases where arthroscopic supervision is used, after debridement of the SCL, the titanium balls can be inserted through a 5.5 mm drill guide and pushed into the bottom of the cyst cavity. Even in the presence of a large communication with the joint, the balls usually attach to the subchondral bone but to prevent migration into the joint a fibrin glue can be applied over the balls
Prognosis for SCLs
Reports evaluating outcome in horses with SCLs of the MFC suggest that older horses, horses with preexisting OA, horses with bilateral lesions, and horses that have upright hindlimb conformation, such as Quarter Horses, have a significantly decreased prognosis for return to soundness following surgical intervention
In one study of horses with MFC SCLs treated with arthroscopic debridement, 64% of young horses became sound while only 34% of mature horses (>3 years) returned to soundness
Following surgical debridement of MFC SCLs, 64% of Thoroughbreds raced, compared with 77% of siblings
There was a difference in racing percentage based on the width of the surface defect: 60.6% of horses with surface debridement of 15 mm or less and 39.3% of horses with surface debridement of at least 15 mm started a race
Amount of cartilage surface involved seemed to be a better predictor of success than depth of the lesion
Polydactyly in Calves
Front limbs generally affected
Radiographs to determine the extent of abnormalities
Flexural and Hyperextension Deformities in Calves
Congenital deformities are seen within 1 or 2 weeks of birth
Other congenital abnormalities sometimes seen simultaneously with flexural deformity are cleft palate, dwarfism, and arthrogryposis
Lupine ingestion by the dam between 30 and 70 days of gestation may result in arthrogryposis
Acquired flexural deformity is seen secondary to reduced weight bearing associated with a primary painful orthopedic disease
Clinical Presentation of Hyperextension Deformities in Calves
Usually seen in newborn calves predominantly affecting the fetlock or carpus and tends to be bilateral unless a congenital malformation of a joint is involved
Hypertension deformity is also seen associated with excessive long-term weight bearing and is often then unilateral
Management of Hyperextension Deformities in Calves
Mild and moderate hyperextension is generally best managed by increased (yet still limited) exercise
If this is not successful at improving the condition within 1 week or the condition is severe, corrective shoeing is used
Heel extension most successful corrective shoeing
If equine glue-on shoe used, both the cuffs of the shoe must be cut (usually dorsally) to prevent compression of the claws
More severe deformity and deformity proximal to the fetlocks should be treated with splints and bandaging
Prognosis usually favorable for all congenital hyperextension deformities without malformation to the bony structures
Clinical Presentation of Flexural Deformities in Calves
Most commonly mild metacarpophalangeal or carpal flexural deformity
Metatarsophalangeal joints involved rarely
Acquired flexural deformity seen in older calves is generally unilateral and secondary to a severe orthopedic injury where the animal cannot bear any or only minimal weight on the affected limb
A dropped fetlock and varus deformity at the carpus of the contralateral limb is evidence of excessive weight bearing
Management of Flexural Deformities in Calves
Mild cases of flexural deformity respond well when placed in housing with good footing
Rather than spending extending periods standing, daily walking exercise is preferable
Medical treatment indicated when no predisposing orthopedic anomaly is present and the limb can be manually extended so the toe's ventral aspect can touch the ground
Splint should be placed on the palmar aspect of the limb, starting at the heel (leaving the claws out) and extending to the proximal MCIII/MTIII (for MCP flexural deformity) or proximal radius (for carpal flexural deformity)
Cast can also be placed and removed/changed 2-3 weeks later
Oxytetracycline can be given to relax the muscles for more rapid correction but should be avoided whenever possible in calves due to nephrotoxicity
Surgical Correction of Flexural Deformities in Calves
Indicated for calves not responding to splinting or with insufficient correction of the deformity to allow weight bearing
Metacarpophalangeal flexural deformity is treated by sequentially transecting the SDFT, DDF, and suspensory ligament until the deformity is released
Tendons of the flexor carpi ulnaris and ulnaris lateralis muscles are transected to treat carpal flexural deformity
Superficial Digital Flexor in Calves
Superficial digital flexor muscle arises from the medial epicondyle of the humerus and divides in two parts, forming two distinct tendons: A deep tendon that passes through the carpal canal and a superficial tendon that passes outside the carpal canal. Both tendons fuse in the midcannon bone but divide at the fetlock into the medial and lateral digit, forming a sleeve that encircles the DDFT. Each divided superficial flexor tendon inserts on the proximal palmar aspect of their respective middle phalanx
Deep Digital Flexor in Calves
DDFT passes into the carpal canal and lays dorsal to the SDFT until near the fetlock, where it divides to insert on the palmar aspect of the distal phalanges of the medial or lateral digit
Suspensory Ligament in Calves
The suspensory ligament (interosseous muscle in young animals) originates from the proximal aspect of the metacarpal bone and divides at the midmetacarpal region, sending a band that joins the superficial flexor tendon. A few centimeters distally, the suspensory ligament divides into three branches
Two abaxial
Further divide distally into two branches that each attach to the corresponding medial and lateral sesamoid bone before continuing to their insertion on the palmar aspect of each proximal phalanx
Each suspensory ligament abaxial branch continues into an extensor branch that joins the abaxial aspect of the extensor tendons on the dorsal aspect of each digit
One middle
Passes through the intertrochlear notch and divides into two branches that each join the axial aspect of the extensor tendons of each digit
Surgical Treatment for Metacarpophalangeal Flexural Deformity in Calves
7.5 cm incision made over lateral (or medial aspect of the DDFT at the level of the midcannon bone
Fascia surrounding the flexor tendon incised in the same plane
SDFT and the connecting branches from the suspensory ligament are identified and elevated with curved hemostats
SDFT and connecting branches from the suspensory ligament transected
Extend the fetlock to assess the degree of correction
If not sufficiently corrected, tendons of the deep digital flexor muscle are isolated and transected
If deformity still not sufficiently corrected, the suspensory ligament is identified immediately caudal to MCIII, isolated with a curved hemostat, and transected
When the superficial digital flexor tendons and their connecting branches from the suspensory ligament are transected, a splint is not needed postoperatively unless tension from the splint is needed to force additional extension for optimum correction
If the deep digital flexor tendons are also transected, the limb(s) may need splint support up to 30 days
If the deep and superficial flexor tendons plus the suspensory ligament are transected, destabilization of the palmar aspect of the carpus occurs
A splint that extends to the radius to give palmar support to the carpus needs to be placed on the back of the limb
Surgical Treatment of Carpal Flexural Deformity in Calves
10 cm incision starting at the accessory carpal bone and extending proximally made on lateral aspect of the carpus over the tendon of the ulnaris lateralis
Incision extended bluntly until the tendons of the ulnaris lateralis and flexor carpi ulnaris tendon are identified, isolated with a curved hemostat, and transected
Splint placed postoperatively on the palmar aspect of the knee unless full correction obtained
Angular and Rotational Limb Deformities in Calves
Common to observe a calf with a valgus deformity and an external rotation
A varus deformity is often associated with internal rotation
Claws usually rotate outward with valgus and inward with varus except in cases of multiple angulations in a limb
Etiology of Angular and Rotational Limb Deformities in Calves
Congenital angular deformity is very rare in cattle and reporedly is in the middiaphysis of the affected long bone when it occurs
Congenital abnormalities most commonly involve multiple joints sometimes with flexural deformities
Growth plate differential growth, commonly seen in horses, is rarely seen in farm animals unless associated with excessive weight bearing where varus deformity is seen
Most calves have a mild carpal valgus deformity of approximately 7 degrees which is within the normal range for most farm animals and does not require treatment
Intermittent pressure allows the growth plate to respond to the line of stress
Partially through reduced blood flow, constant pressure reduces longitudinal growth from the affected physis plate
The uncompressed side of the physis maintains normal growth which results in an angular deformity (usually varus)
Often seen at the hock or carpus on the contralateral limb (limb without a painful orthopedic problem)
Clinical Presentation of Angular and Rotational Limb Deformities in Calves
Although mild valgus deformity is relatively common, varus deformity is abnormal. If varus deformity is found unilaterally, the contralateral limb should be examined for a significant orthopedic injury as a cause for excessive weight bearing in the deformed limb/joint
Obtaining radiographic evaluation is important in investigation of orthopedic injuries, which are often important causal factors in angular deformity in farm animals
Diagnosis of Angular and Rotational Limb Deformities in Calves
DP view needed for examination of the anatomic location of the deformity and its measurement
Medical Management of Angular and Rotational Limb Deformities in Calves
Trimming the claws of a young calf creates growth plate response to stress applied opposite the deformity so self correction occurs
Trimming and other hoof manipulation is based on the principle that the hoof will turn in the direction of the longer claw or toward the side of the wider wall
The lateral claw must not extend more than 1 cm, otherwise the stress on the lamina may cause inflammation and pain
Acrylic or a shoe can also be applied to the claw to extend the lateral or medial claw
Surgical Management of Angular and Rotational Limb Deformities in Calves - Transphyseal Screws
Transphyseal screws are placed across the growth plate to retard growth on the convexed side of the deformity
1 cm stab incision made midway 2-3 cm above the growth plate (except for the hock where a stab incision is made at the most distal and extraarticular area of the appropriate malleolus)
Pilot hole drilled with a 2.5 or 3.2 mm drill bit
One self-tapping screw, 3.5 or 4.5 mm (usually 32-36 mm in length) is placed across the ipsilateral third of the growth plate
Implant removed as soon as the leg is acceptably straightened
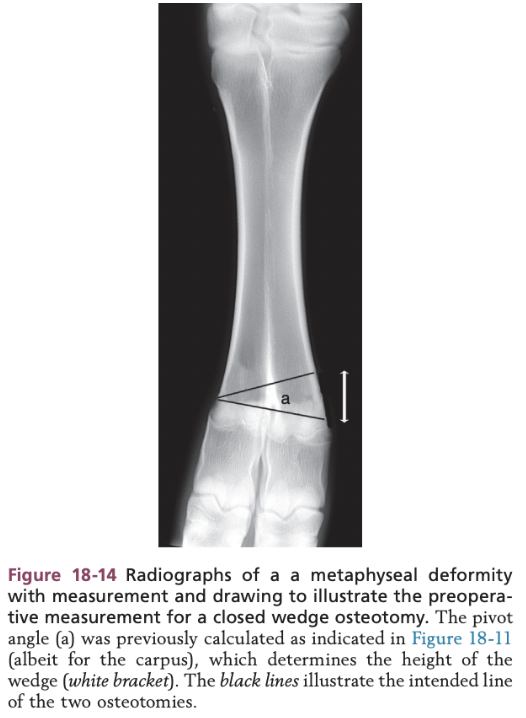
Surgical Management of Angular and Rotational Limb Deformities in Calves - Closing Wedge Osteotomy
Line is drawn parallel to the joint at the level of the pivot point
Height of the second osteotomy site is calculated by using the angle of deviation at the pivot point
Surgical Management of Angular and Rotational Limb Deformities in Calves - Step-Wise Osteotomy
Horizontal osteotomy line drawn parallel to the joint, starting at the pivot line but extending only through half the diameter of the affected bone in the dorsopalmar (plantar) plane)
Line is drawn from the axial end of the horizontal osteotomy line and extends 5 cm proximally along the long axis of the bone
Second vertical line the same length is drawn from the same starting point but angled to represent the previously measured pivot angle, the width of that wedge is measured
The last osteotomy line is drawn horizontally from the proximal aspect of these two vertical lines and extending perpendicular to the long axis of the proximal bone
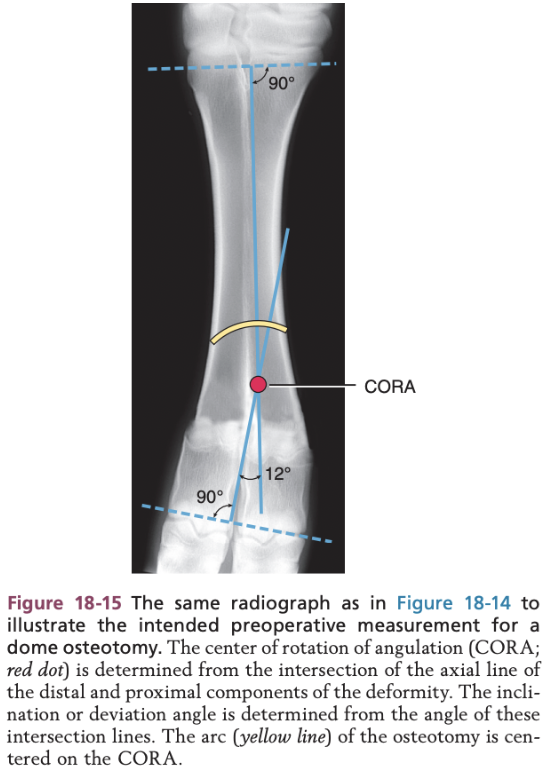
Surgical Management of Angular and Rotational Limb Deformities in Calves - Cylindrical (i.e. Dome) Osteotomy
First the pivot point is identify and is referred to as the center of rotation of angulation (CORA)
Angle of the deformity is determined from the intersection of the drawn line
Dome or curve osteotomy is performed centered on the CORA
This allows two curved bone ends to angulate and translate on each other to correct the deformity with maximal bone contact and the limb length is maintained because no bone is removed
Approach to Osteotomies in Calves
Skin incision made over the dorso or dorsolateral aspect of the affected long bone
Inverted V-shaped tenotomy of the common (or long) digital flexor tendon done exposing the diaphysis
Incision extended to the affected bone and subperiosteal dissection exposes the bone before the osteotomies
2 mm Kirschner wire placed 2 cm proximal to the joint below and 2 cm distal to the joint above at 90 degrees to the long axis of the bone to be osteotomized to facilitate realignment of the bone ends after the osteomy
Surgical Techniques in Calves - Closing Wedge Osteotomy
Using a reciprocating saw, affected bone transected parallel to the joint surface immediately distal to the level of the pivot point
Height of second osteotomy is measured from the radiographs
Starting on the convex side, the second osteotomy is extended to the opposite side of the bone until it meets the first osteotomy site
Bone fragments fixed after wedge is removed
Surgical Techniques in Calves - Step-Wedge Ostectomy
3.2 mm hole drilled from dorsal to palmar (plantar) in the center of the bone at the intended start of the longitudinal osteotomy lines
Second hole drilled 5 cm proximal
Two holes prevent inadvertent longitudinal fissures associated with the creation of the longitudinal osteotomies
Oscillating saw or Gigli wire used to join the two holes by a longitudinal osteotomy
Using the width measurement of the wedge needed, another hole placed proximally and second longitudinal osteotomy is performed
Horizontal osteotomies are made by cutting the bone parallel to the joint without extending any further than the distal aspect of the longitudinal osteotomies
Proximal osteotomy site is done perpendicular to the long axis of the proximal fragment
Distal osteotomy is made parallel to the distal joint
Lag screws are applied across the vertical component created as part of the internal fixation repair
Surgical Techniques in Calves - Cylindrical or Dome Osteotomy
Arc osteotomy using a biradial saw with the calculated arc of a circle centered on CORA
Prognosis for Angular and Rotational Limb Deformities in Calves
Reasonable for angular deformities associated with growth plate imbalance, such as most valgus deformities
Wedge osteotomies carry a fair prognosis for functionality, although a cosmetic defect due to enlargement at the surgery site is expected
Prognosis for angular deformity secondary to contralateral orthopedic injury is generally poor because it is usually centered over a joint and is also dependent on the prognosis of the primary orthopedic injury