1F: ATP, Water and Ions
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
62 Terms
All organisms require a constant supply of _______ to maintain their cells and stay alive.
energy
What do organisms require energy for?
for anabolic reactions, building larger molecules from smaller molecules
to move substances across the cell membrane (active transport) or to move substances within the cell
In some/all known forms of life, ATP (adenosine triphosphate) from respiration is used to…
all
…transfer energy in all energy-requiring processes in cells.
This is why ATP is known as the…
…universal energy currency.
ATP is a ___________ _________.
ATP is a phosphorylated nucleotide.
Adenosine (a nucleoside) can be combined with how many phosphorylated groups?
What are the names of these compounds?
one, two or three
One phosphate group = adenosine monophosphate (AMP)
Two phosphate groups = adenosine diphosphate (ADP)
Three phosphate groups = adenosine triphosphate (ATP)
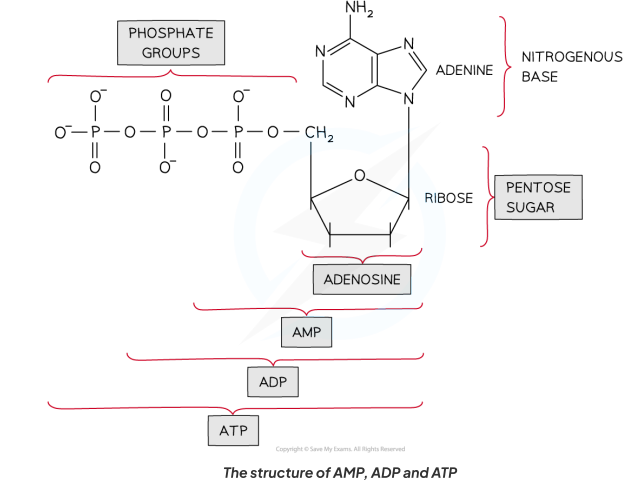

Do you need to know the full structural formula of AMP, ADP and ATP?
No - just their components: a pentose sugar, adenine (a nitrogenous base), and the number of phosphate groups.
Adenine is a ____, while adenosine is a _________ .
Adenine is a base, while adenosine is a nucleoside (adenine + sugar).
How does ATP release energy?
Energy released during the reactions of respiration is transferred to the molecule ATP.
As ADP forms, free energy is released that can be used for processes within a cell, e.g. DNA synthesis.
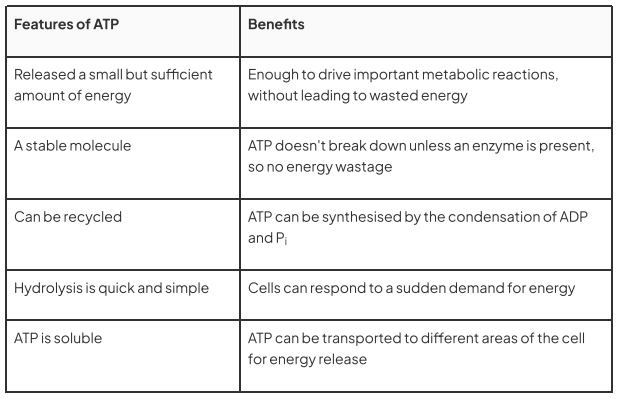
Why is the use of ATP as an energy-currency beneficial?
The hydrolysis of ATP can be carried out quickly and easily wherever energy is required within the cell by the action of just one enzyme, ATPase
A useful (not too small, not too large) quantity of energy is released from the hydrolysis of one ATP molecule - this is beneficial as it reduces waste but also gives the cell control over what processes occur
ATP is relatively stable at cellular pH levels
Hydrolysis of ATP to ADP and an inorganic phosphate group (Pi) is catalysed what the enzyme?
ATP hydrolase, sometimes called 'ATPase'
The hydrolysis of ATP can be coupled to which energy-requiring reactions within cells?
the active transport of ions up a concentration gradient
enzyme-controlled reactions that require energy
muscle contraction and muscle fibre movement
When ATP is hydrolysed, what is formed?
ADP
Pi
What can the inorganic phosphate released during the hydrolysis of ATP be used for?
To phosphorylate other compounds, often making them more reactive.
What are the features of ATP, and what are the benefits of each of these?

Can you use energy annd ATP interchangably in an exam?
What phrases should be used?
No. Use…
“use ATP”
“requiring ATP hydrolysis”
…when talking about reactions that need energy.
Which phrases should you avoid when alking about reactions that produce ATP?
“produce energy”
Define energy.
The capacity to do work and cannot be created or destroyed.
Define ATP.
A molecule that stores and transports chemical energy within cells.
How much ATP do humans use in a day on average?
50 kg
What is the maximum amount of ATP in the human body at any given time?
What does this show us?
200g
This shows that organisms cannot build up large stores of ATP, and it rarely passes through the cell surface membrane.
This means the cells must make ATP as and when they need it.
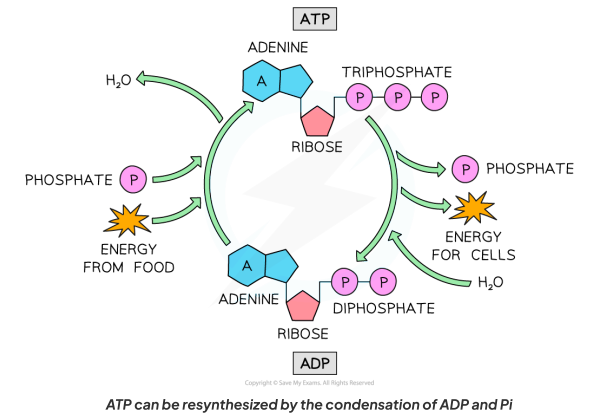
When is ATP formed?
Give the key information about this reaction.
When ADP is combined with an inorganic phosphate (Pi) group by the enzyme ATP synthase.
This reaction requires energy
Water is released as a waste product, therefore, ATP synthesis is a condensation reaction
ATP is made during the reactions of _________ and ___________.
All of an animal's ATP comes from __________.
ATP is made during the reactions of respiration and photosynthesis.
All of an animal's ATP comes from respiration.
Give a diagram showing the connversion of ATP to ADP, and vice-versa.

What are the types of ATP synthesis?
ATP can be made in two different ways:
Substrate-linked phosphorylation (occurs in the glycolysis stage of respiration)
Chemiosmosis (occurs in the electron transport chain stage of respiration)
Why is water of great biological importace?
Itis the medium in which all metabolic reactions take place in cells.
Water is composed of atoms of ________ and _______.
Water is composed of atoms of hydrogen and oxygen.
What are some key properties to remember about water?
polar
hydrogen bonds form between water molecules
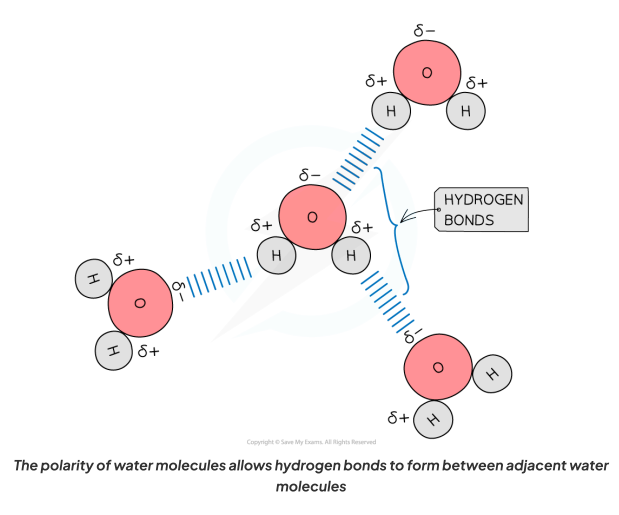
Why do hydrogen bonds form between water molecules?
As a result of the polarity of water, hydrogen bonds form between the positively and negatively charged regions of adjacent water molecules.
Hydorgen bonds contribute to which properties of water?
An excellent solvent – many substances can dissolve in water
A relatively high specific heat capacity
A relatively high latent heat of vaporisation
Water is less dense when a solid
Water has high surface tension and cohesion
It acts as a reagent
These all make water so important to living organisms.
Water is a ________ in many metabolic reactions, including ___________ and _________ reactions.
Water is a metabolite in many metabolic reactions, including condensation and hydrolysis reactions.
What are condensation reactions?
In condensation reactions, smaller molecules combine to form a larger molecule, with the removal of a water molecule.
What are examples of condensation reactions?
formation of peptide bonds between amino acids to make proteins
formation of glycosidic bonds in carbohydrates
formation of ester bonds in lipids
What are hydrolysis reactions?
In hydrolysis reactions, water is added to break a bond within a larger molecule, splitting it into smaller units.
What are examples of hydrolysis reactions?
breaking proteins into amino acids
breaking starch into glucose
breaking triglycerides into fatty acids and glycerol
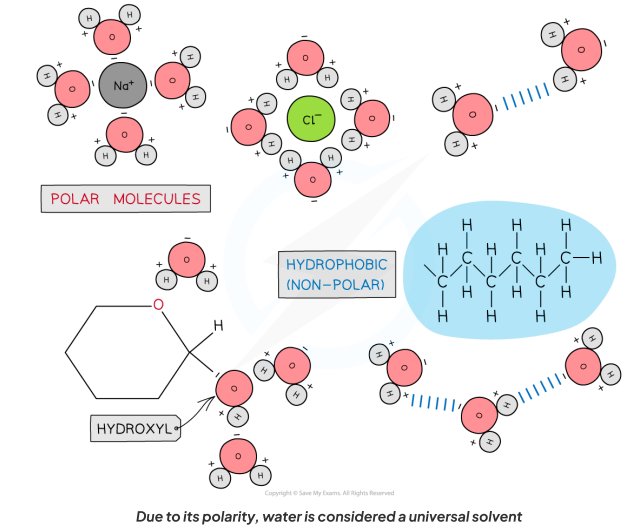
What is water a solvent for? Why?
As water is a polar molecule, many ions (e.g. sodium chloride) and covalently bonded polar substances (e.g. glucose) will dissolve in it.
What is the benefit of water being a solvent?
This allows chemical reactions to occur within cells (as the dissolved solutes are more chemically reactive when they are free to move about)
Metabolites can be transported efficiently (except non-polar molecules, which are hydrophobic)

What is specific heat capacity?
A measure of the energy required to raise the temperature of 1 kg of a substance by 1°C.
What is water’s specific heat capacity?
It has a high specific heat capacity of 4200 J / Kg C, meaning a relatively large amount of energy is required to raise its temperature
Why does water have a high specific heat capacity?
Due to the many hydrogen bonds present in water.
It takes a lot of thermal energy to break these bonds and a lot of energy to build them; thus, the temperature of water does not fluctuate greatly.
What is the benefit of water’s high specific heat capacity?
stable habitats can be provided, especially for aquatic organisms
water absorbs lots of heat with minimal temperature change
This helps maintain stable internal and external temperatures, essentialfor enzyme function
water in blood plasma transfers heat around the body, aiding temperature regulation
As blood flows through warmer tissues, it absorbs heat without large temperature shifts
What is water’s latent heat of vapourisation?
To change state (from liquid to gas), a large amount of thermal energy must be absorbed by water to break the hydrogen bonds and evaporate.
What is the benefit of water’s high latent heat of vapourisation?
This is an advantage for living organisms, as only a little water is required to evaporate for the organism to lose a great amount of heat.
This provides a cooling effect for living organisms, e.g. the transpiration from leaves or the evaporation of water in sweat on the skin.
Describe the cohesion between of water.
Hydrogen bonds between water molecules allow for strong cohesion between water molecules.
What is the benefit of water molecules’ cohesion?
This allows columns of waterto move through the xylem of plants and the blood vessels in animals.
This also enables surface tension where a body of water meets the air; these hydrogen bonds occur between the top layer of water molecules to create a sort of film on the body of water
Describe the adhesion of water.
Water is also able to hydrogen bond to other molecules, such as cellulose, which is known as adhesion.
What is the benefit of water molecules’ adhesion?
This also enables waterto move up the xylem due to transpiration.
What key things are important to remember when answering exam questions about water?
It is important to know where the hydrogen bonds form between water molecules (the oxygen of one water molecule to the hydrogen atom of another).
Also, when discussing the role water has in living organisms, remember to mention the ‘why’ in relation to its properties (i.e. it is an excellent solvent because of the polar nature of water molecules)
What is an ion?
An atom (or sometimes a group of atoms) that has an electrical charge.
What is a cation?
An ion that has a positive charge.
What is an anion?
An ion that has a negative charge.
What is an inorganic ion?
An ion that does not contain carbon.
Where do we find inorganic ions?
In solution in the cytoplasm and body fluids of organisms.
The concentration of certain ions can __________ and can be used in cell ___________ and __________ _____________.
The concentration of certain ions can fluctuate and can be used in cell signalling and neuronal transmission.
What inorganic ions do we need to know the properties and roles of?
hydrogen
iron
sodium
phosphate
What should we know about hydrogen ions?
They are protons (H+)
The concentration of H+ in a solution determines the pH
The concentration of H+ is therefore very important for enzyme-controlled reactions, which are all affected by pH
How does hydrogen ion concentration determine pH?
There is an inverse relationship between the pH value and the hydrogen ion concentration.
The more H+ ions present, the lower the pH (the more acidic the solution)
The fewer H+ ions present, the higher the pH (the more alkaline the solution)
Why is the concentration of H+ very important for enzyme-controlled reactions?
The fluids in the body normally have a pH value of approximately 7.4
The maintenance of this normal pH is essential for many of the metabolic processes that take place within cells
Changes in pH can affect enzyme structure
How many versions of iron ions are there?
There are two oxidation states:
Iron (II) ions, also known as ferrous ions (Fe2+)
Iron (III) ions, also known as ferric ions (Fe3+)
What should we know about iron ions?
Iron ions are essential as they can bind oxygen
Haemoglobin is the large protein in red blood cells that is responsible for transporting oxygen around the body
Haemoglobin is made up of four polypeptide chains that each contain one Fe2+
This Fe2+ is a key component in haemoglobin as it binds to oxygen
What should we know about sodium ions?
Sodium ions (Na+) are required for the transport of glucose and amino acids across cell surface membranes (e.g. in the small intestine)
Glucose and amino acid molecules can only enter cells (through carrier proteins) alongside Na+ in a process known as co-transport
Na+ is also required for the transmission of nerve impulses
What should we know about phosphate ions?
Phosphate ions (PO43-) attach to other molecules to form phosphate groups, which are an essential component of DNA, RNA and ATP
In DNA and RNA,the phosphate groups allow individual nucleotides to bond to form polynucleotides
In ATP, the bonds between phosphate groups store energy
These phosphate groups can be easily attached or detached
When the bonds between phosphate groups are broken, they release a large amount of energy, which can be used for cellular processes
Phosphates are also found in phospholipids, which are key components of the phospholipid bilayer of cell membranes