Adaptive Platform Clinical Trials
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
The standard clinical trial
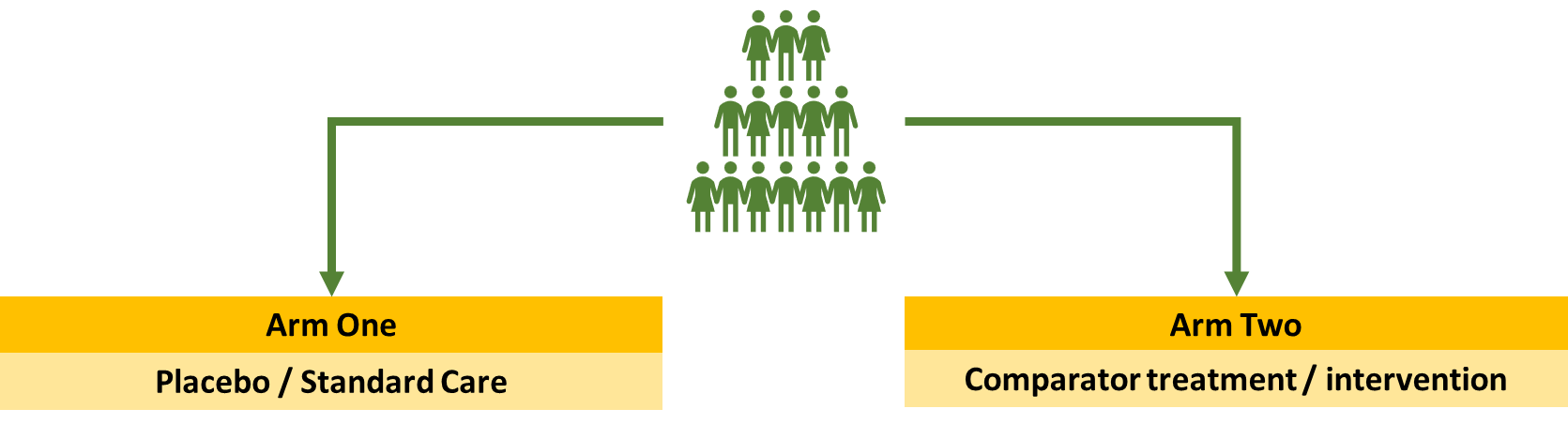
You take a group of people and randomise them into arm 1 which is the placebo or the comparator treatment. These trials are intervention focused as they look at impact of different treatments for a disease in a specific population
The standard clinical trial
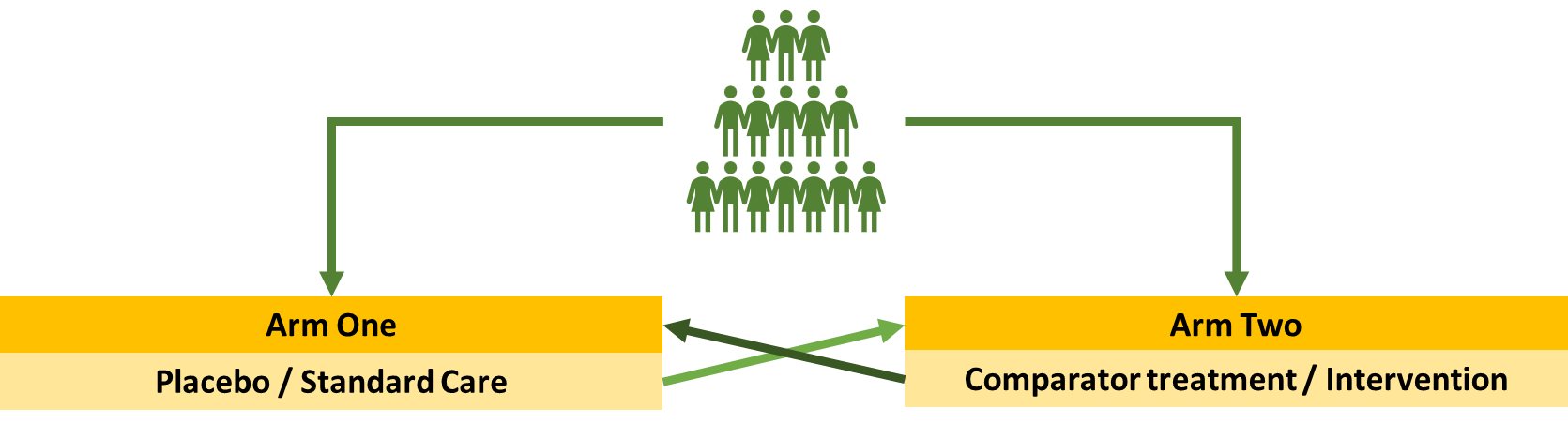
This is a crossover trial where each population will spend a certain amount of time in each one before swapping over -
Adaptive Platform Trial
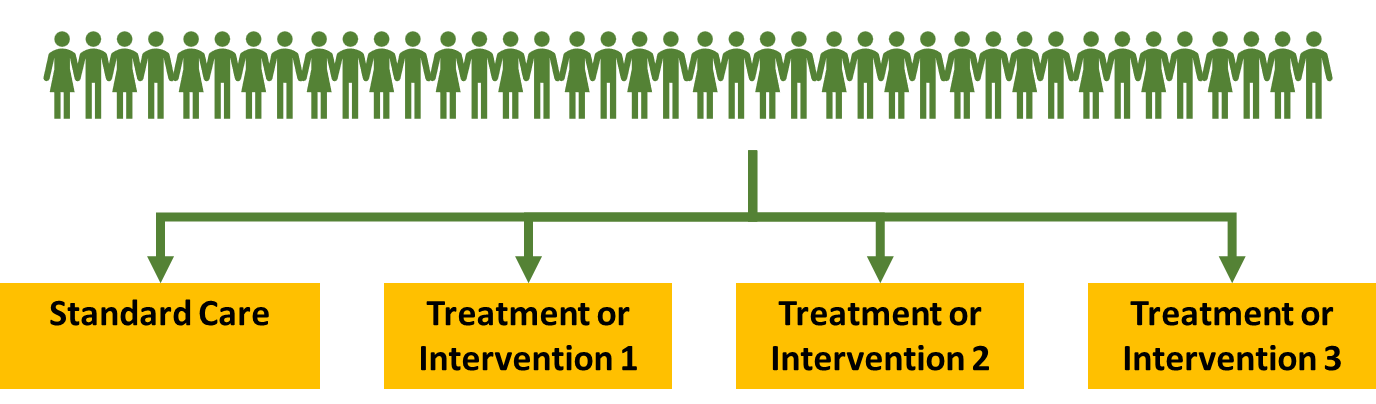
Modern clinical trials/blockbuster trials are now using adaptive platform trial format. This is where you take a much bigger and diverse population that represents the real world. It has multiple arms. Instead of doing a one to one randomisation - there may be more people in the placebo arm allowing you to compare results more effectively.
Adaptive Platform Trial
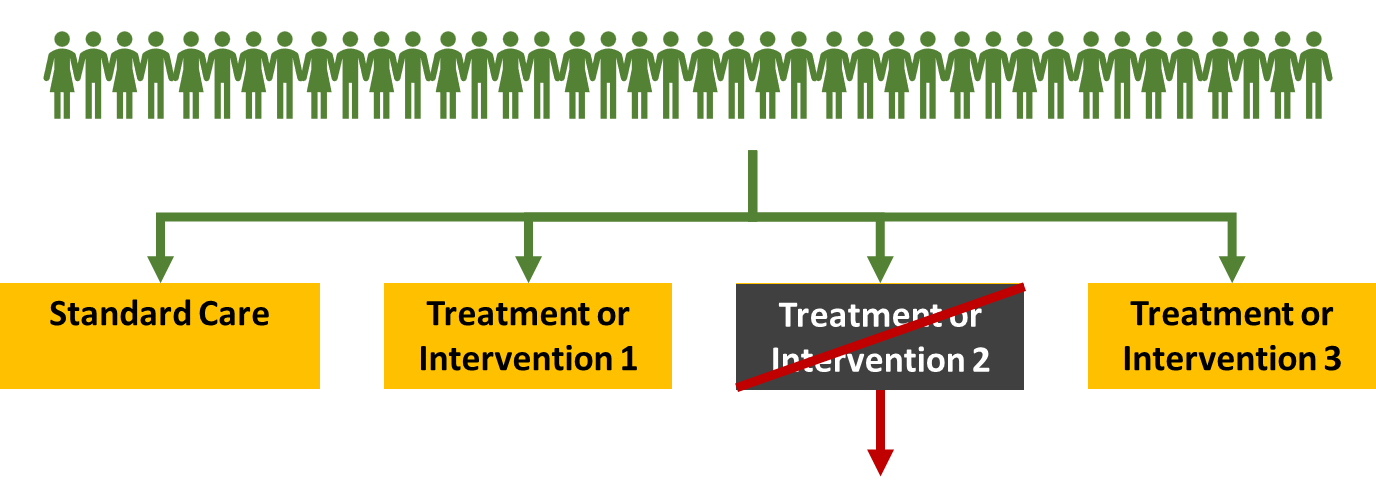
Allows you to be flexible with how you run the trial
For example if you found that intervention 2 was causing more harm than good then it wouldn’t really be ethical to keep enrolling people into this population so you could close that arm. You can still continue enrolling people into the other arms. This means you won’t lose the data for the standard care arm and can use it to compare to the other treatments to see if its beneficial
Adaptive Platform Trial
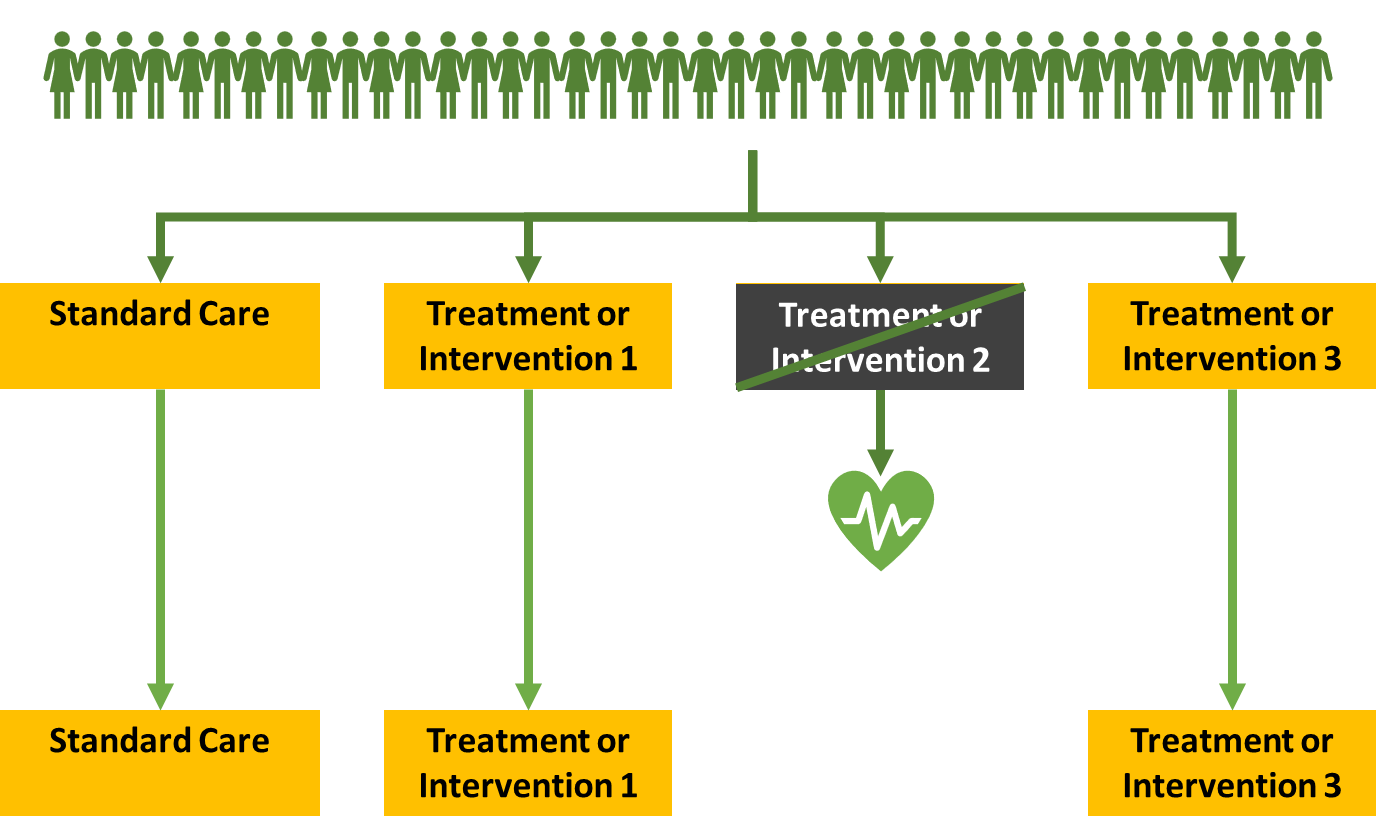
If intervention 2 was found to have worked well you can close it because you know that it provides benefit already. You can then focus on the other arms
Adaptive Platform Trial
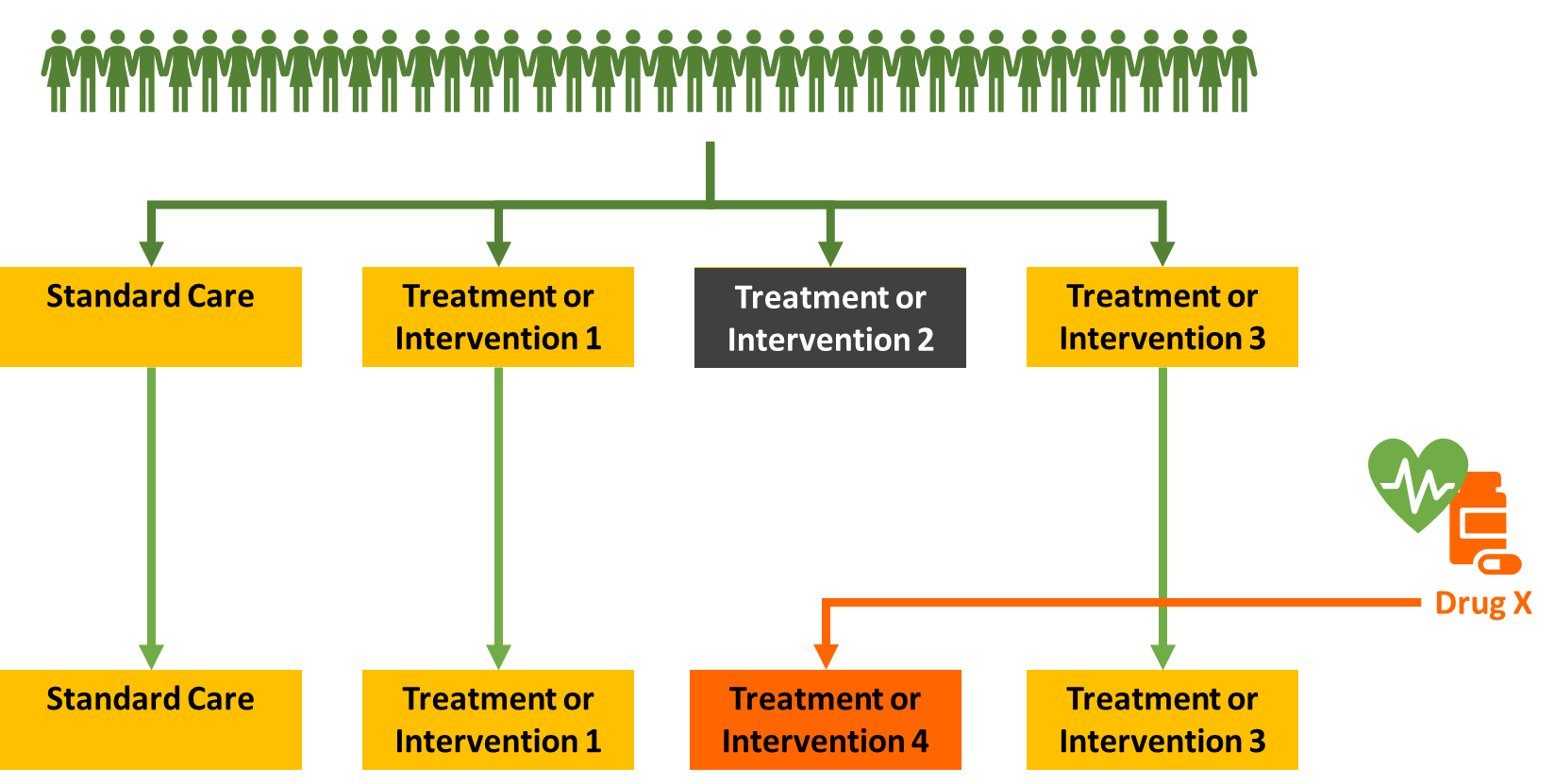
Adaptive Platform Trial
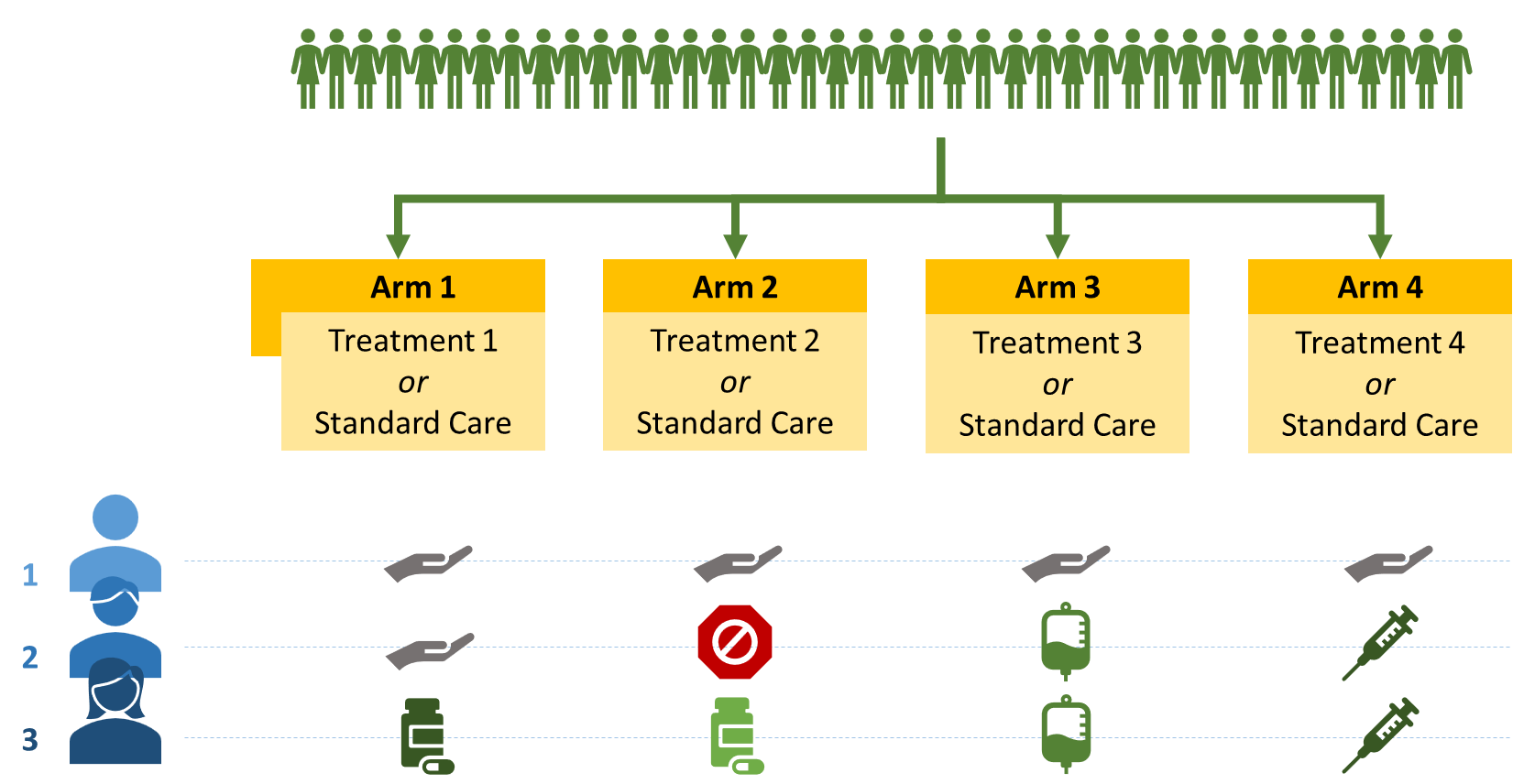
Patient 1 was randomised into standard care for all for arms
Patient 3 randomised to all 4 treatments
Patient 2 was unsuitable for treatment 2 - becomes a standard care essentially.
Allows you to see if combination of drugs are better than just one
downside is you need a lot more patients to show a significant difference.
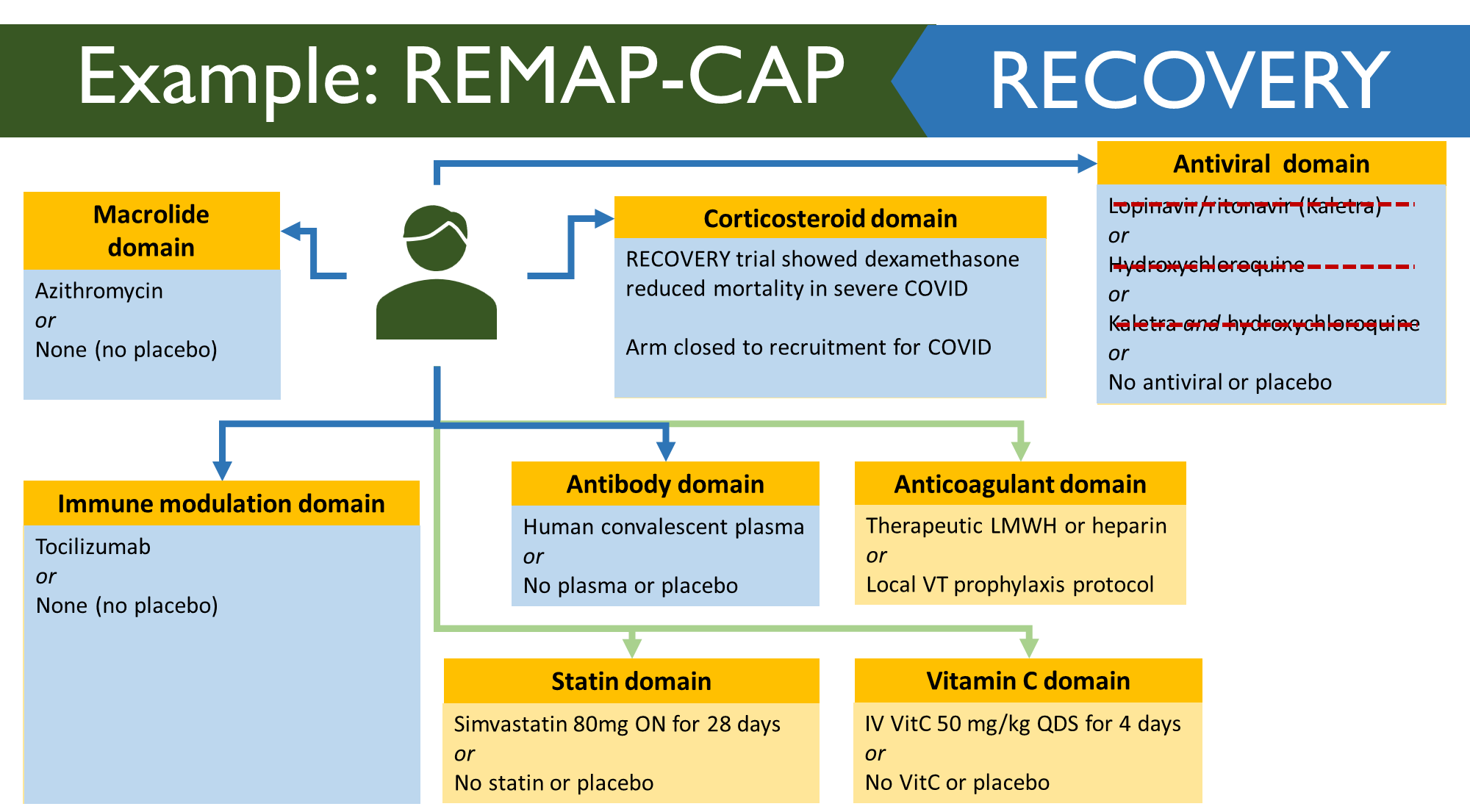
Example: REMAP-CAP
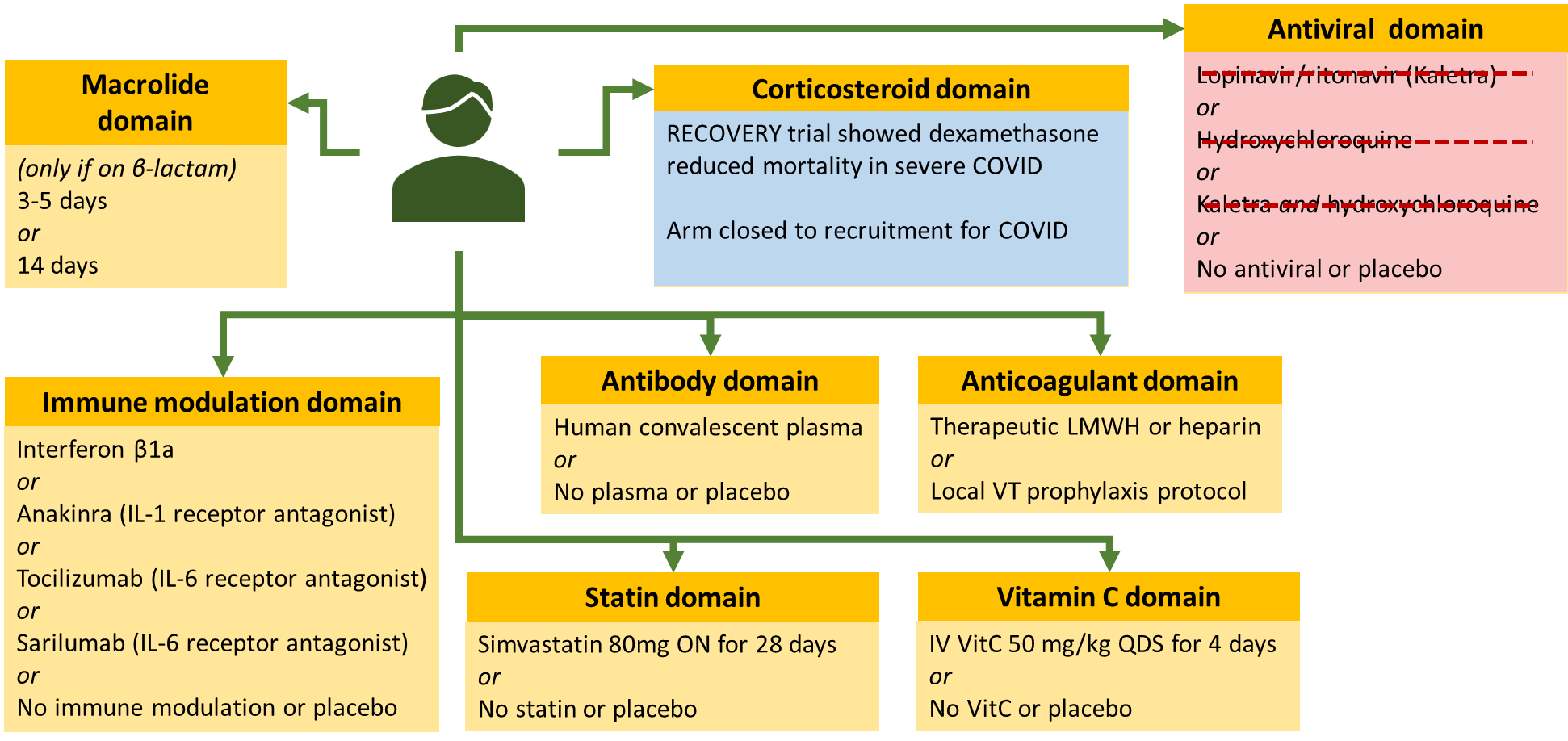
This is a multifactorial adaptive trial and is looking at community acquired pneumonia. These are more disease focused compared to standard trials. When COVID came it was causing viral pneumonia in some patients. However, this is different to normally CAP. To start they took 2 domains from the original trials and applied it to the COVID one (Macrolide and corticosteroid domain). Not a lot of people in the macrolide one as they could only enter if they were given beta lactam for the disease but obviously COVID is viral. Leaves you with corticosteroid arm – compared low dose, high dose or placebo. 4 new arms were introduced. Then statin domain was introduced as these patients already on statins tended to do better. Then Vitamin C was introduced as it was shown to improve outcomes. Then the antiviral domain didn’t get very far. Koletra was the first one to go – supply wasn’t easy. Also a lot of these patients required NG tube and koletra cannot be given through this. Hydrochlorquine was found to be harmful or not beneficial. MHRA ruled that it needs to stop. Corticosteroid one – stopped enrolling people as it found that dexamethasone is effective and in this trial hydrocortisone was being used.
REMAP RECOVERY
Domains in recovery studies were much smaller. Allowed hospitals to see if they should enrol in both trials or just one.