Structure & Bonding (IGCSE Edexcel Chemistry)
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
Metallic Bonding
Strong electrostatic force of attraction between the sea of delocalised electrons and positive metal ions
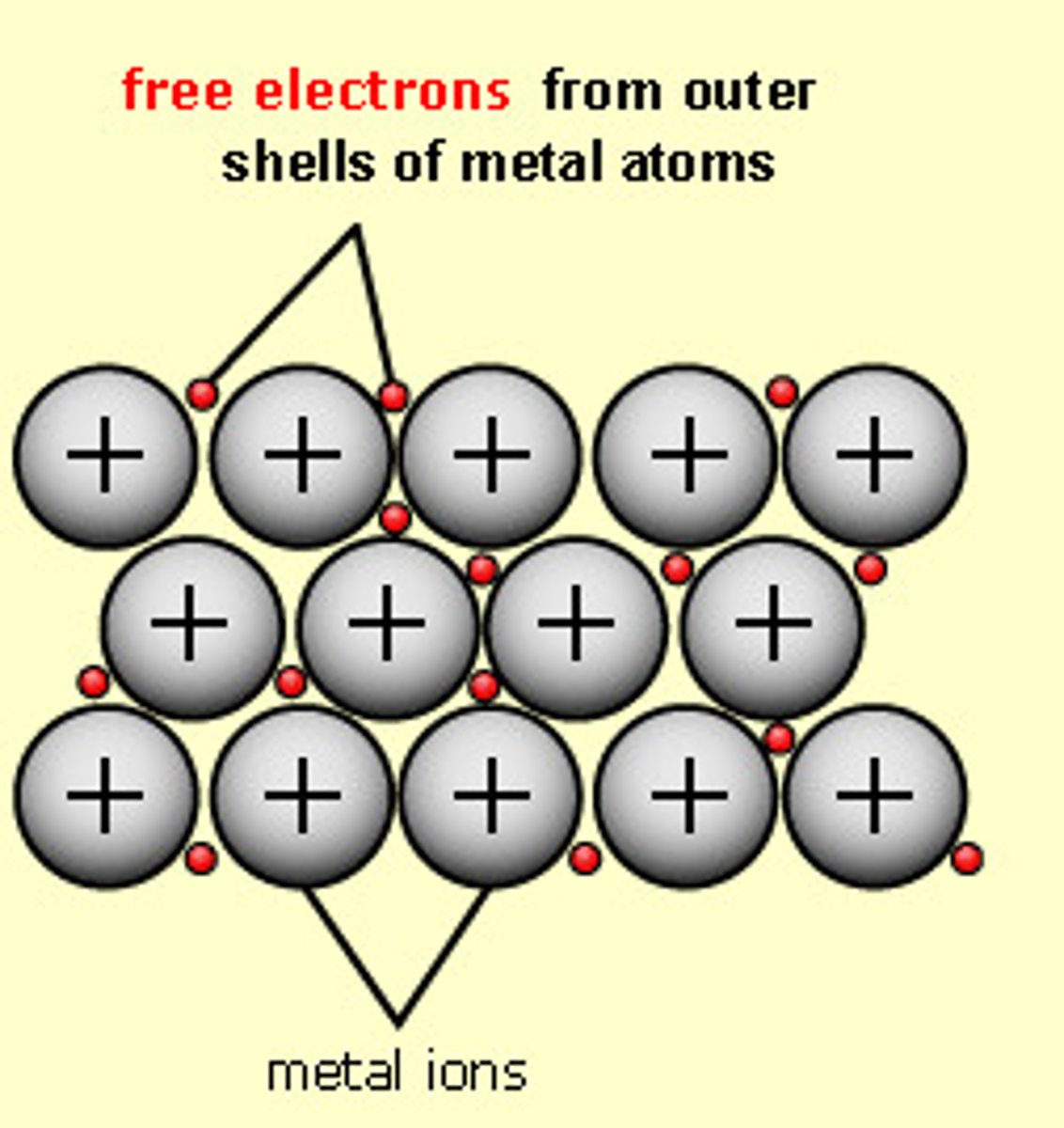
Malleable
easy to shape or bend
Ions
positively and negatively charged atoms which are formed after electrons are lost / gained
Ammonium ion
NH₄⁺
Carbonate ion
CO₃²⁻
Sulphate ion
SO₄²⁻
Nitrate ion
NO₃⁻
Hydroxide ion
OH⁻
Ionic Bonding
Strong electrostatic force of attraction between oppositely-charged ions
Giant ionic lattice
Structure formed when ions attract each other in a 3D cube
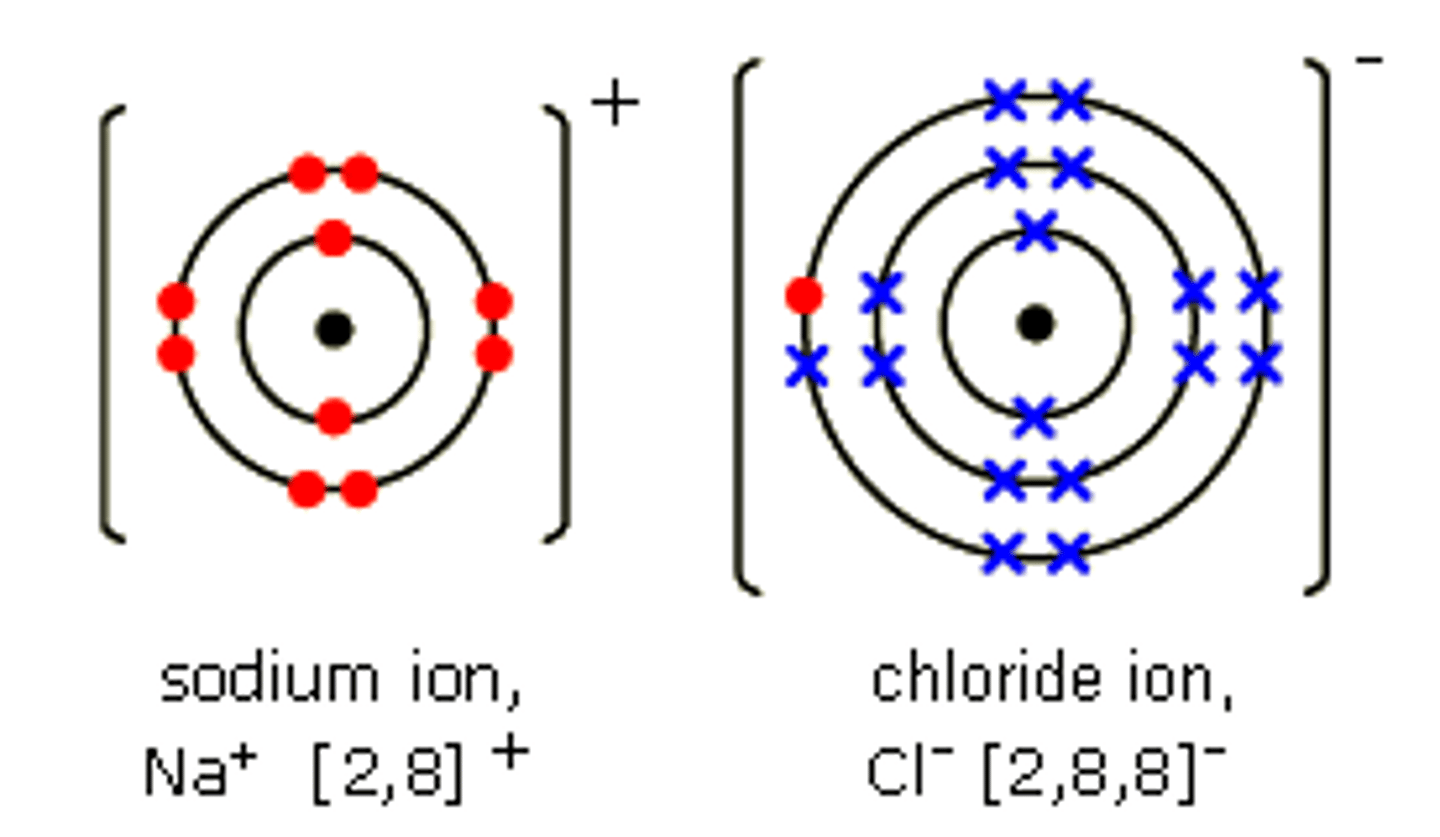
Dot cross diagram for NaCl
Dot cross diagram for sodium oxide
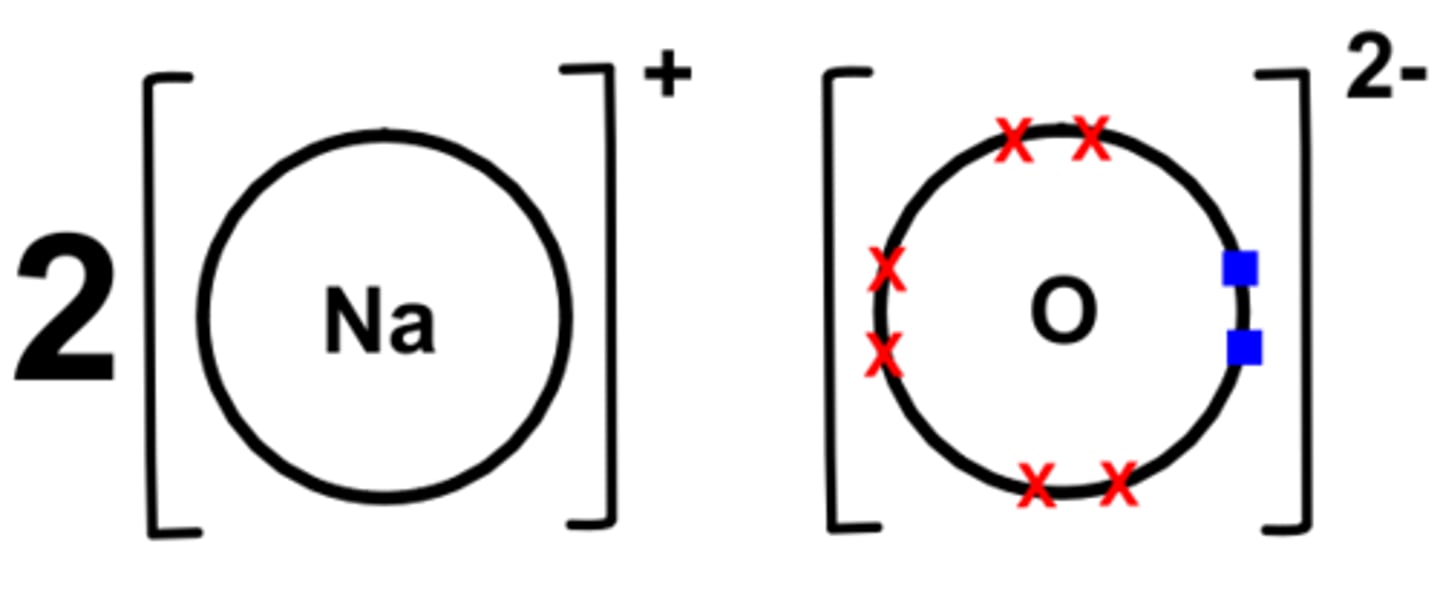
Dot cross diagram for magnesium chloride
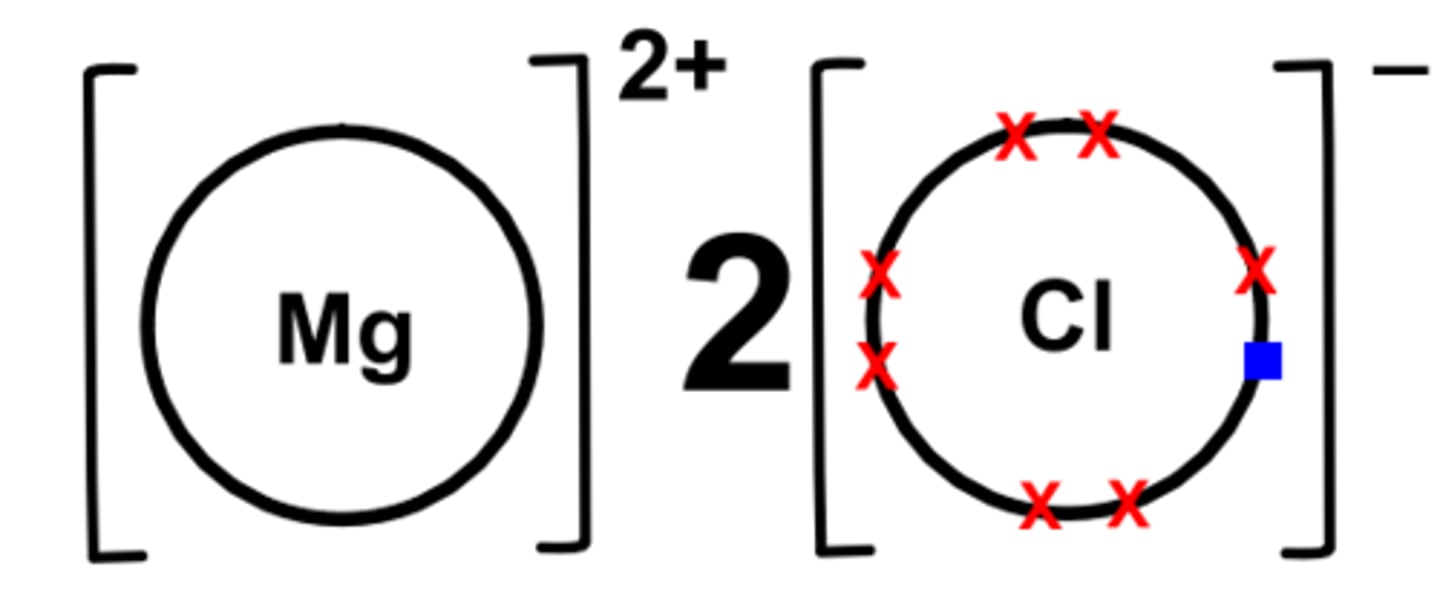
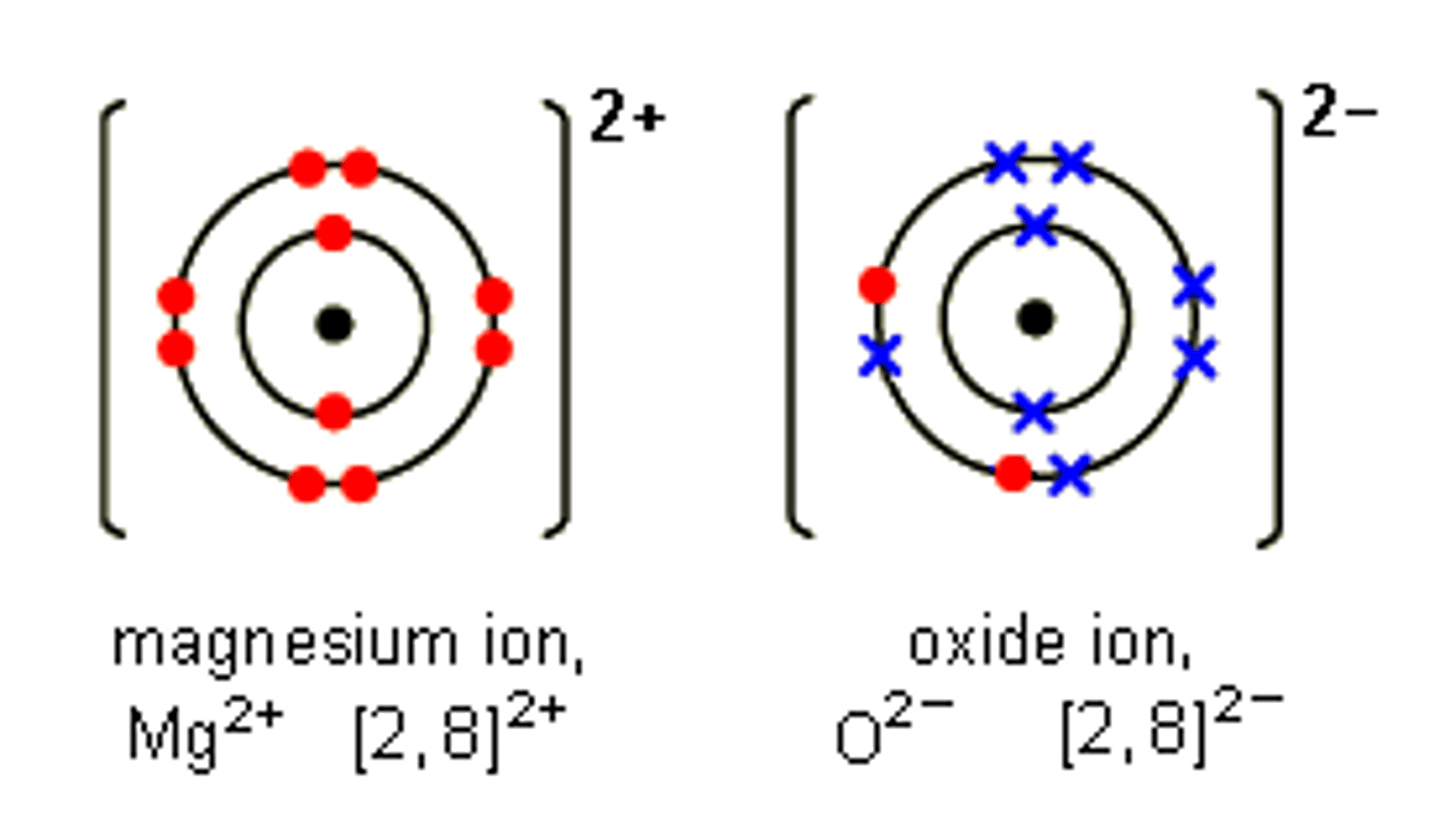
Dot cross diagram for magnesium oxide
Covalent bonding
Strong electrostatic force of attraction between the shared pair of electrons and the nuclei of atoms
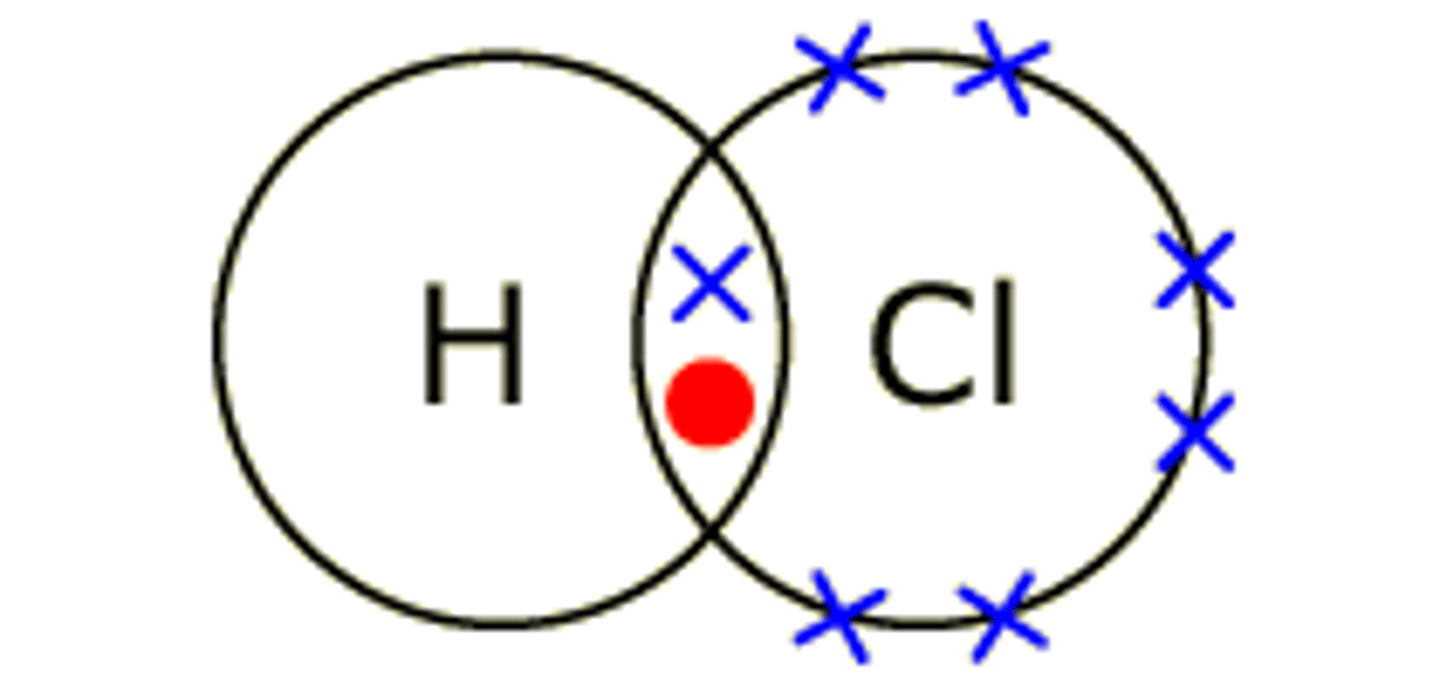
Dot cross diagram for hydrogen chloride
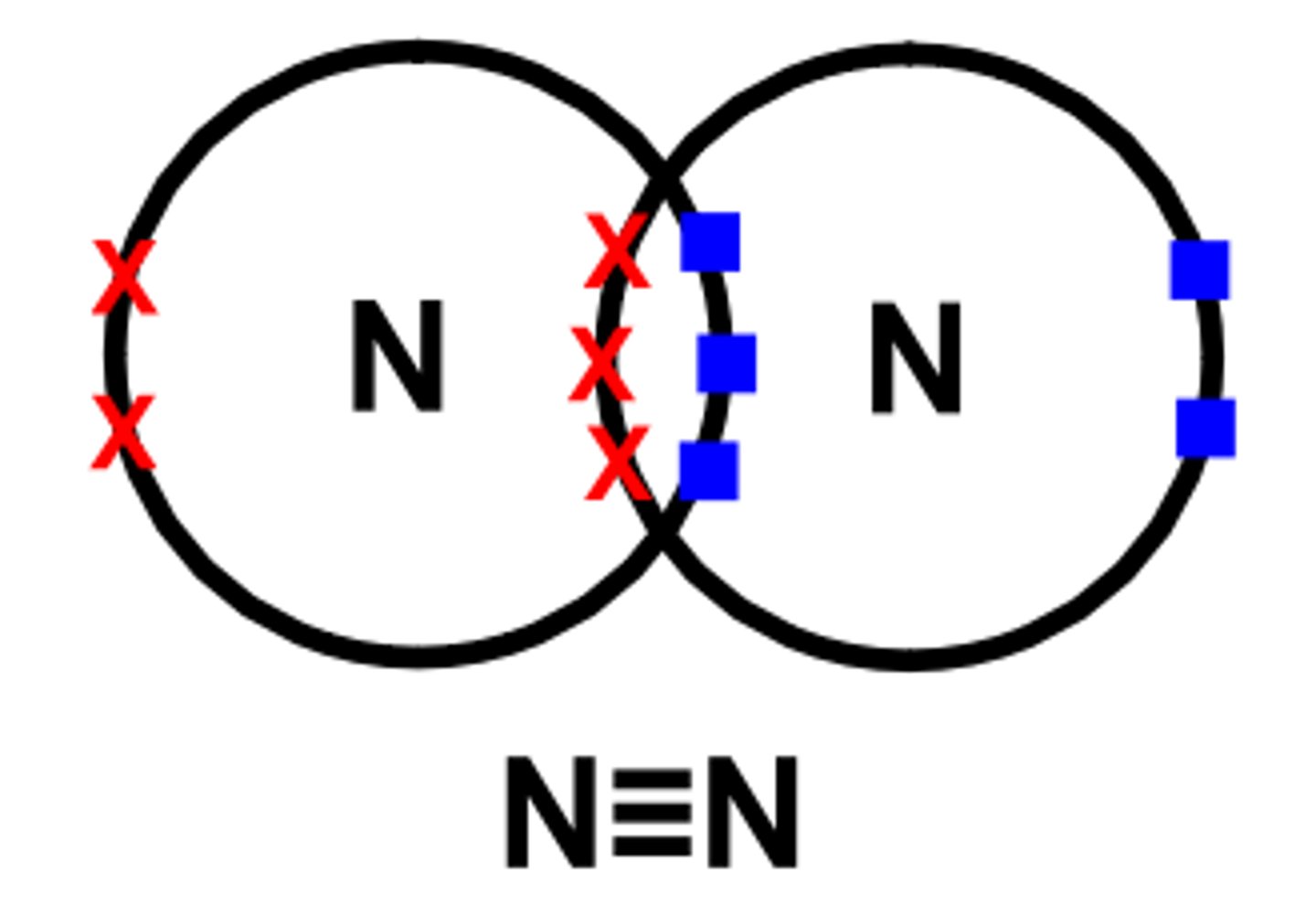
Dot cross diagram for nitrogen molecule
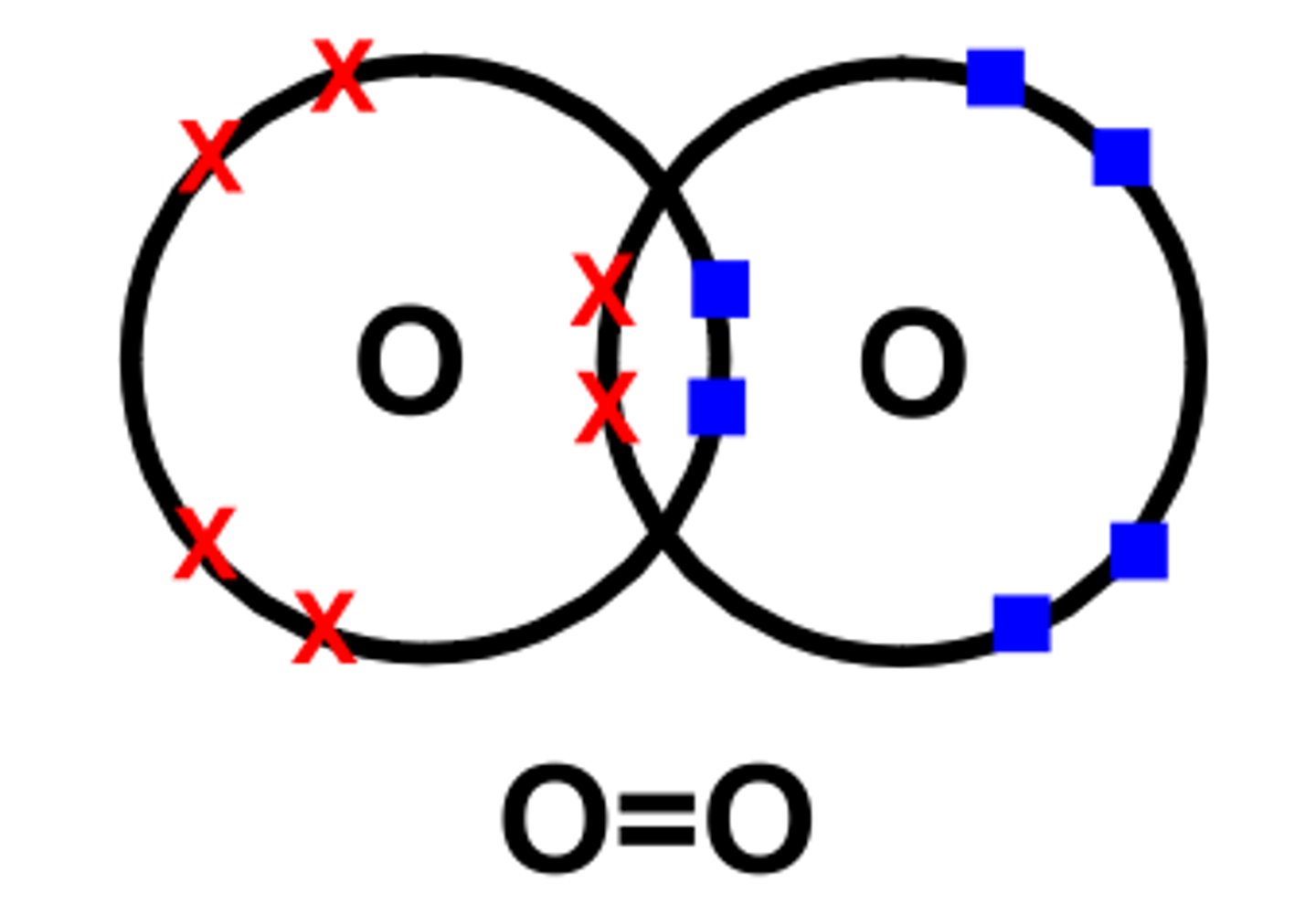
Dot cross diagram for oxygen molecule
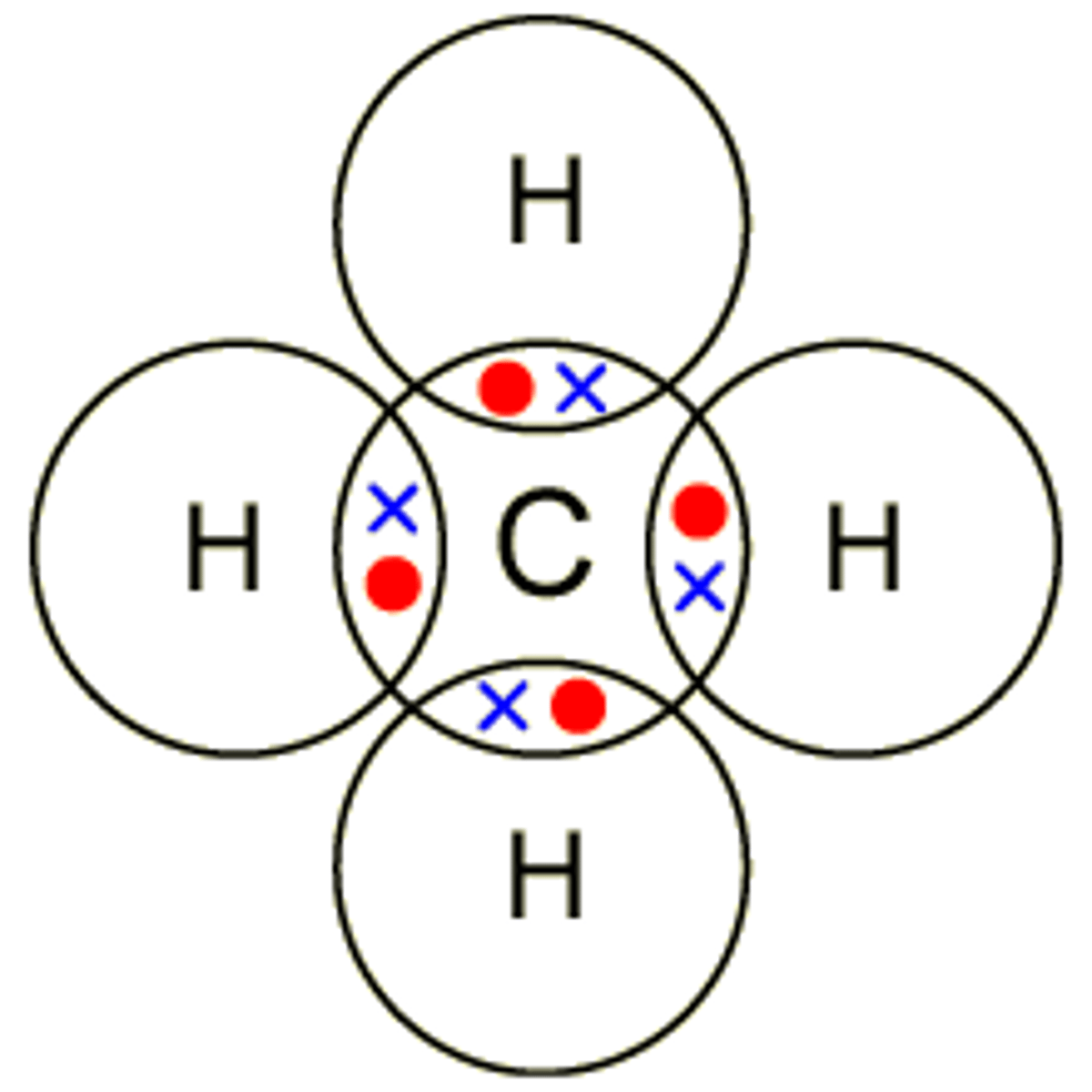
Dot cross diagram for methane
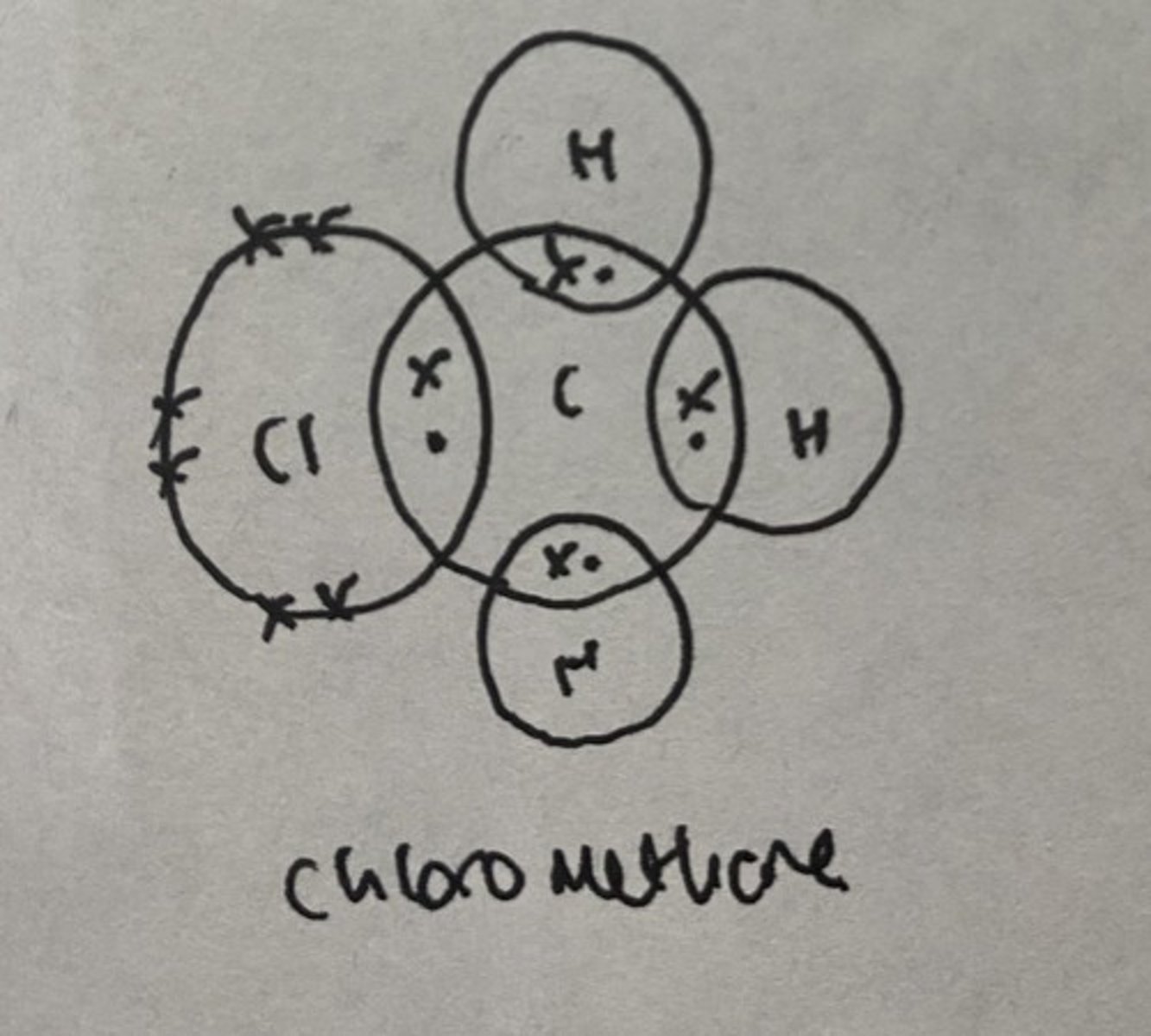
Dot cross diagram for chloromethane
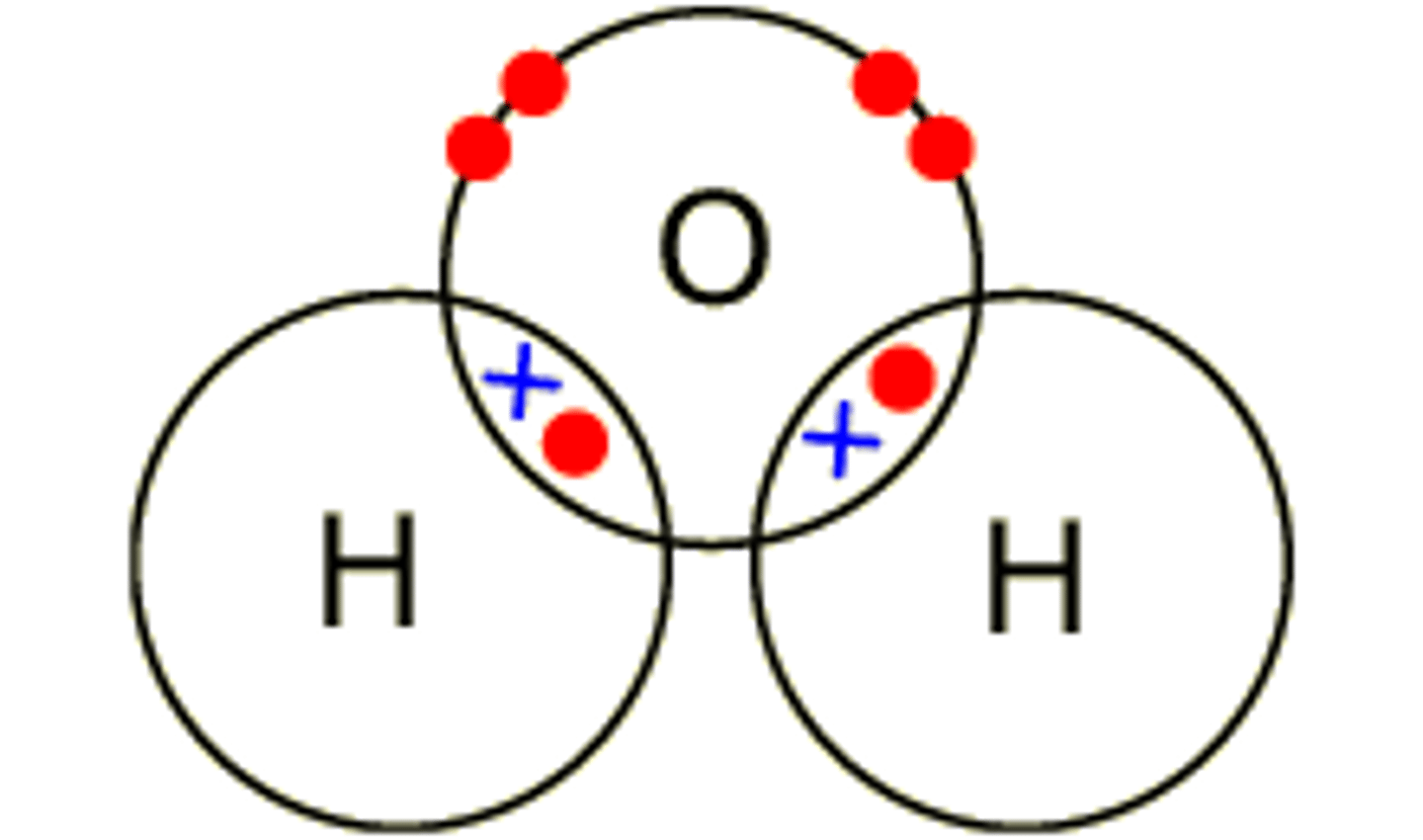
Dot cross diagram for water
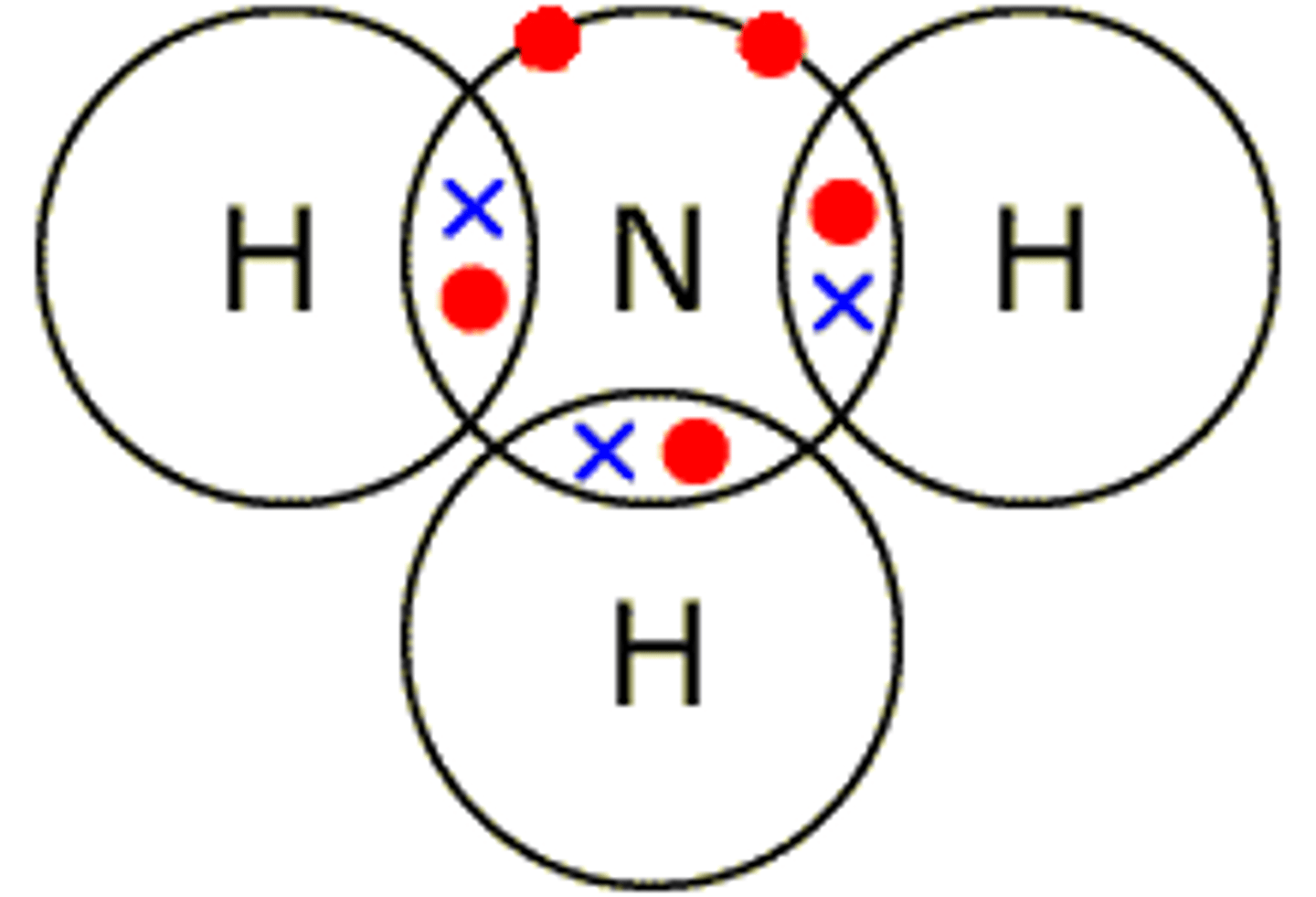
Dot cross diagram for ammonia
Dot cross diagram for ethane
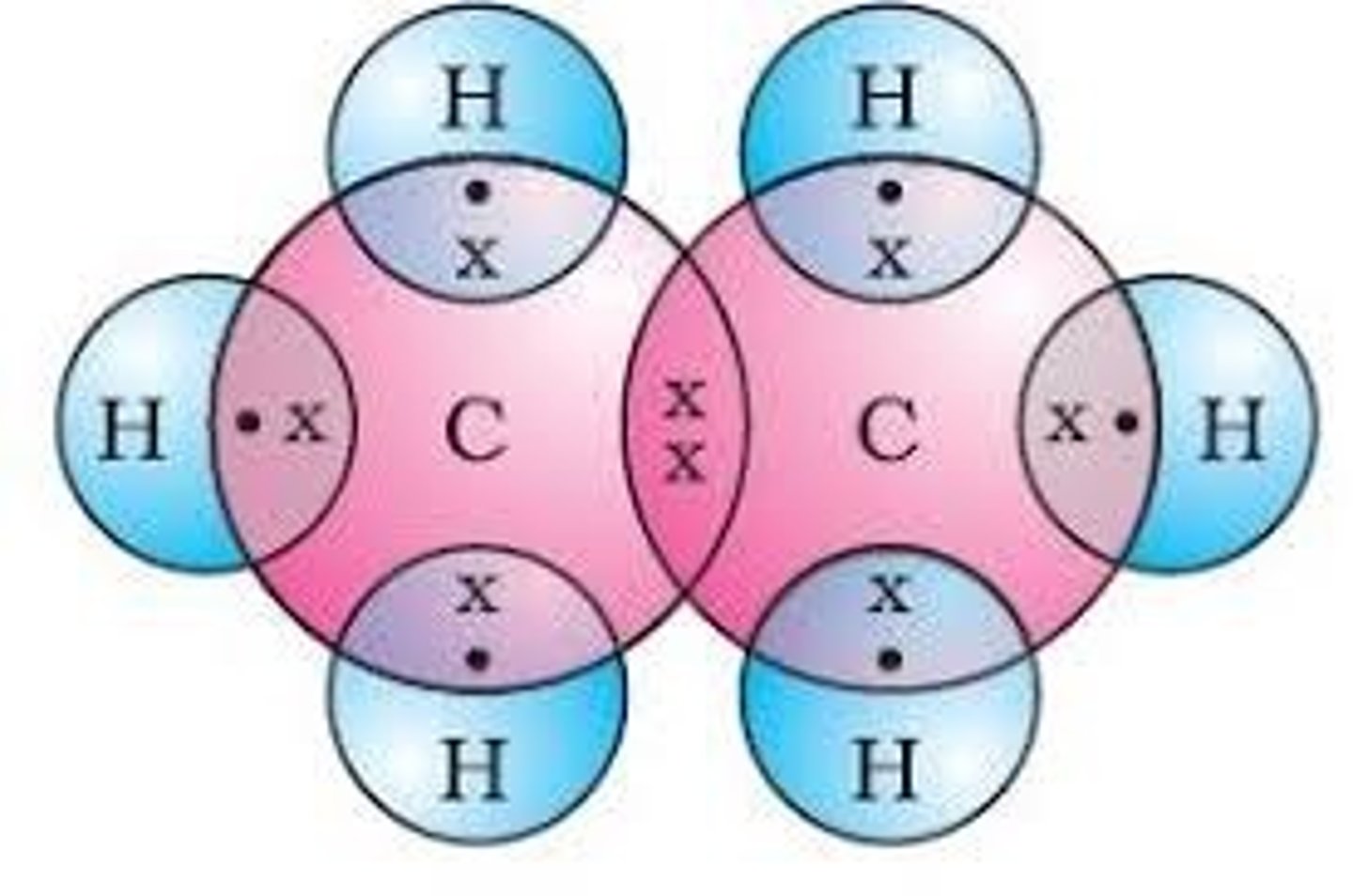
Dot cross diagram for ethene
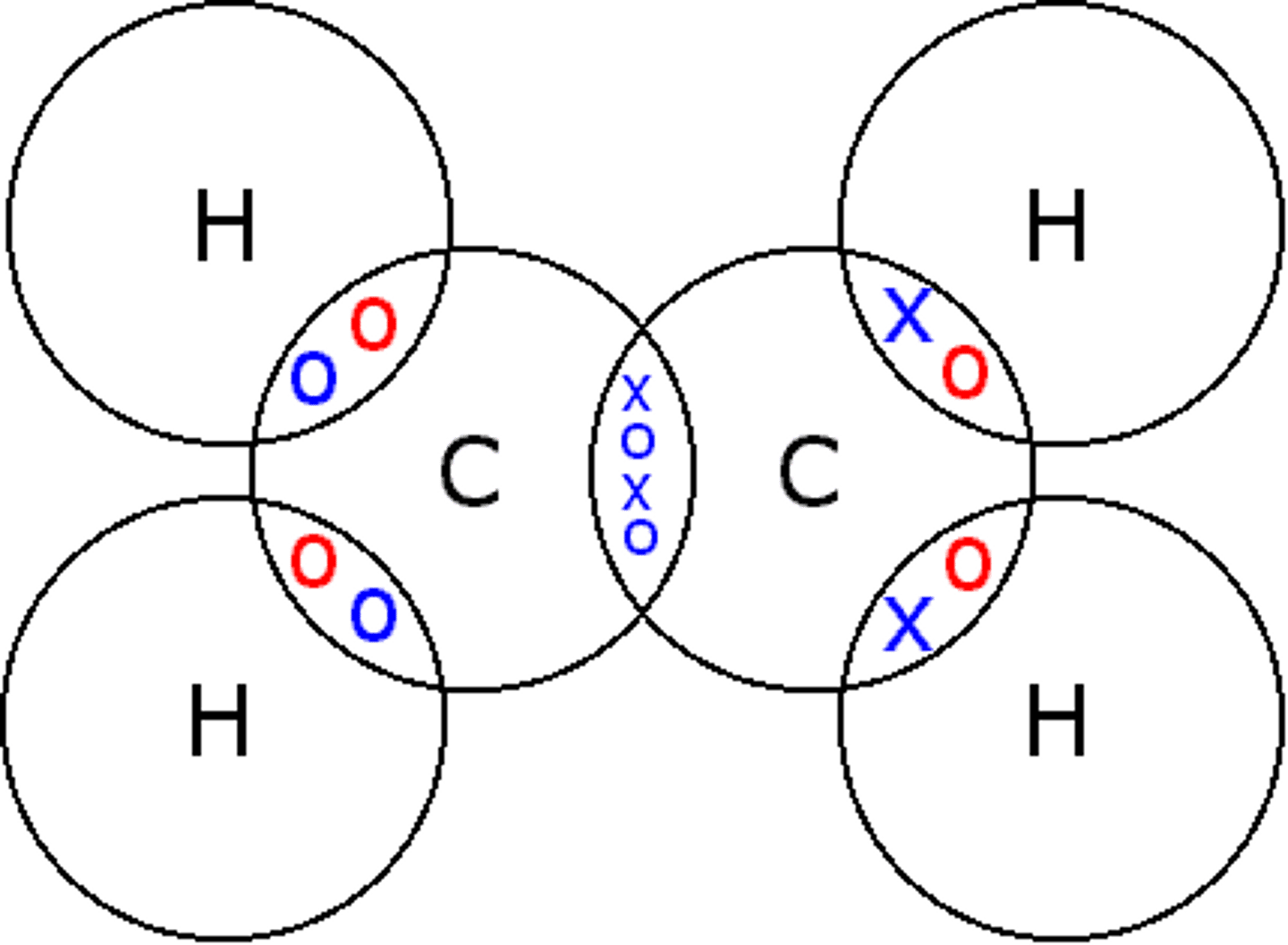
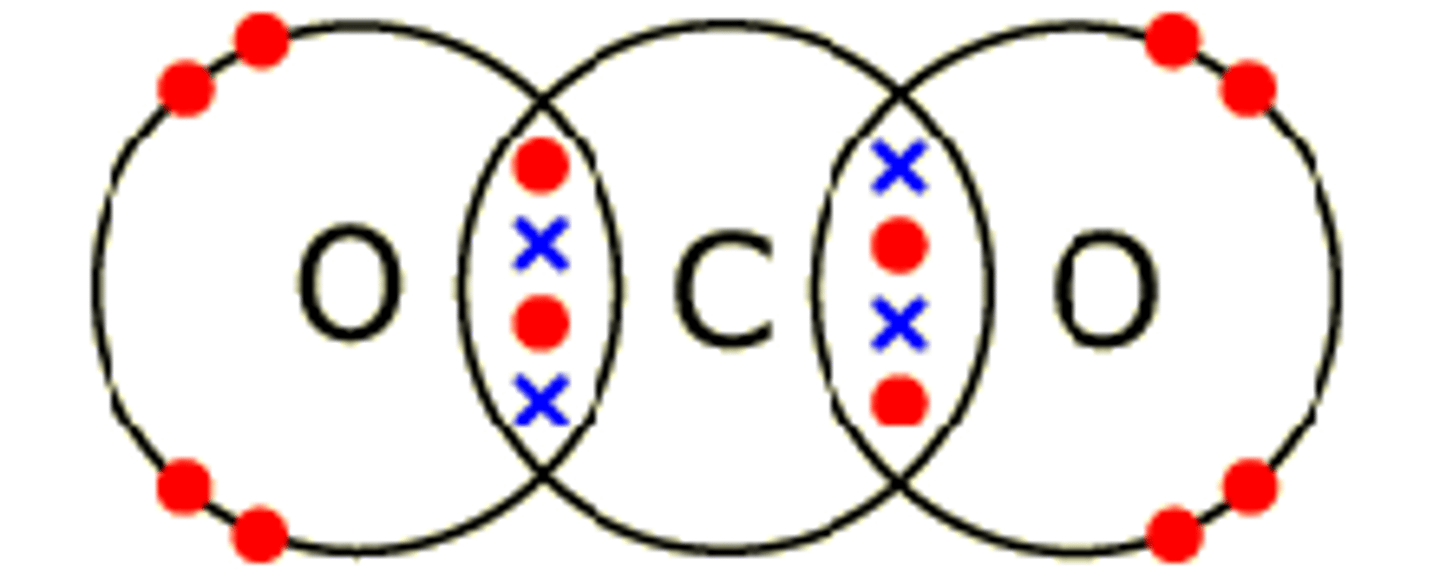
Dot cross diagram for carbon dioxide
Reason why covalent molecules have low boiling points
Not much energy is needed to break the weak intermolecular forces between molecules
Diamond structure
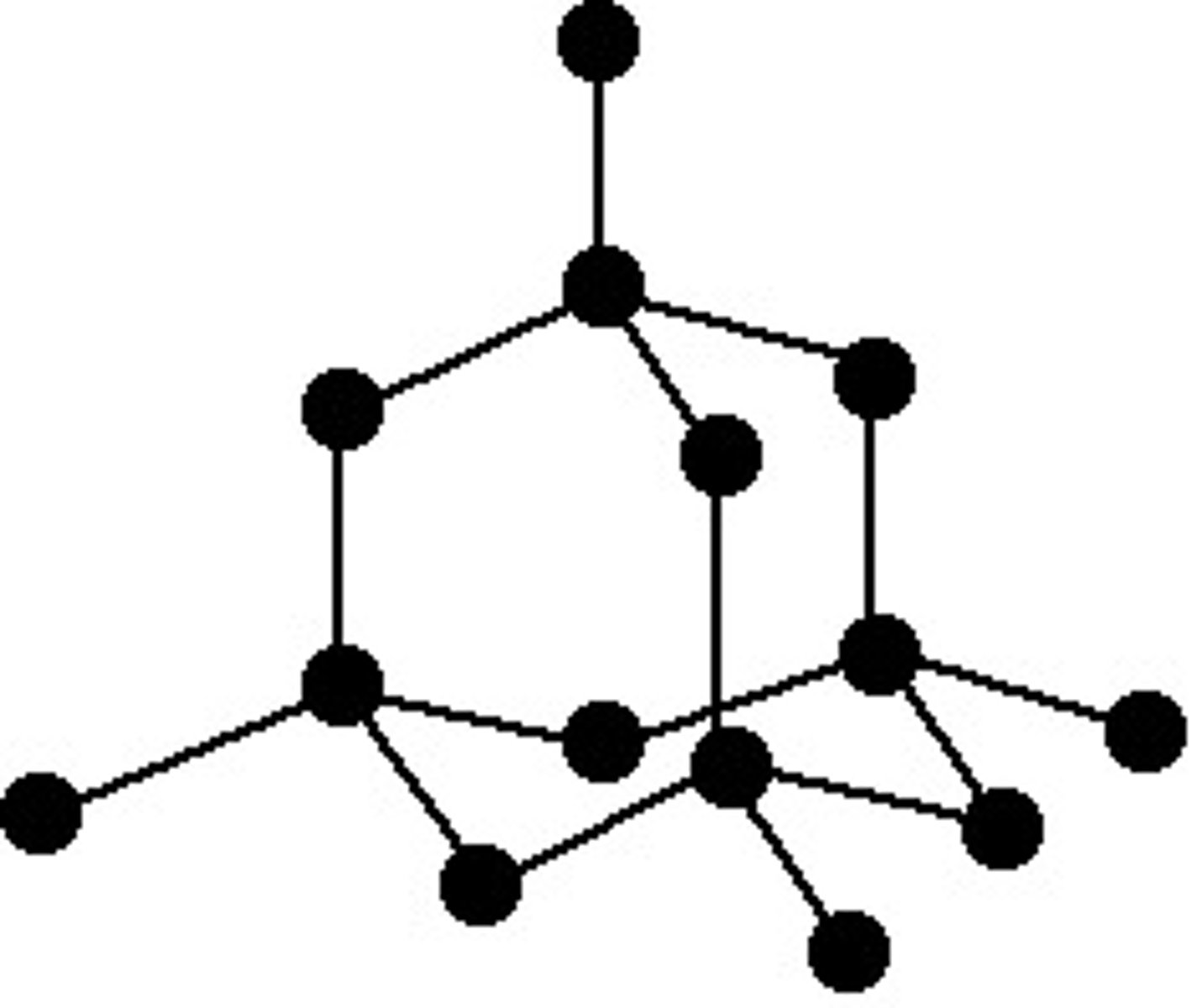
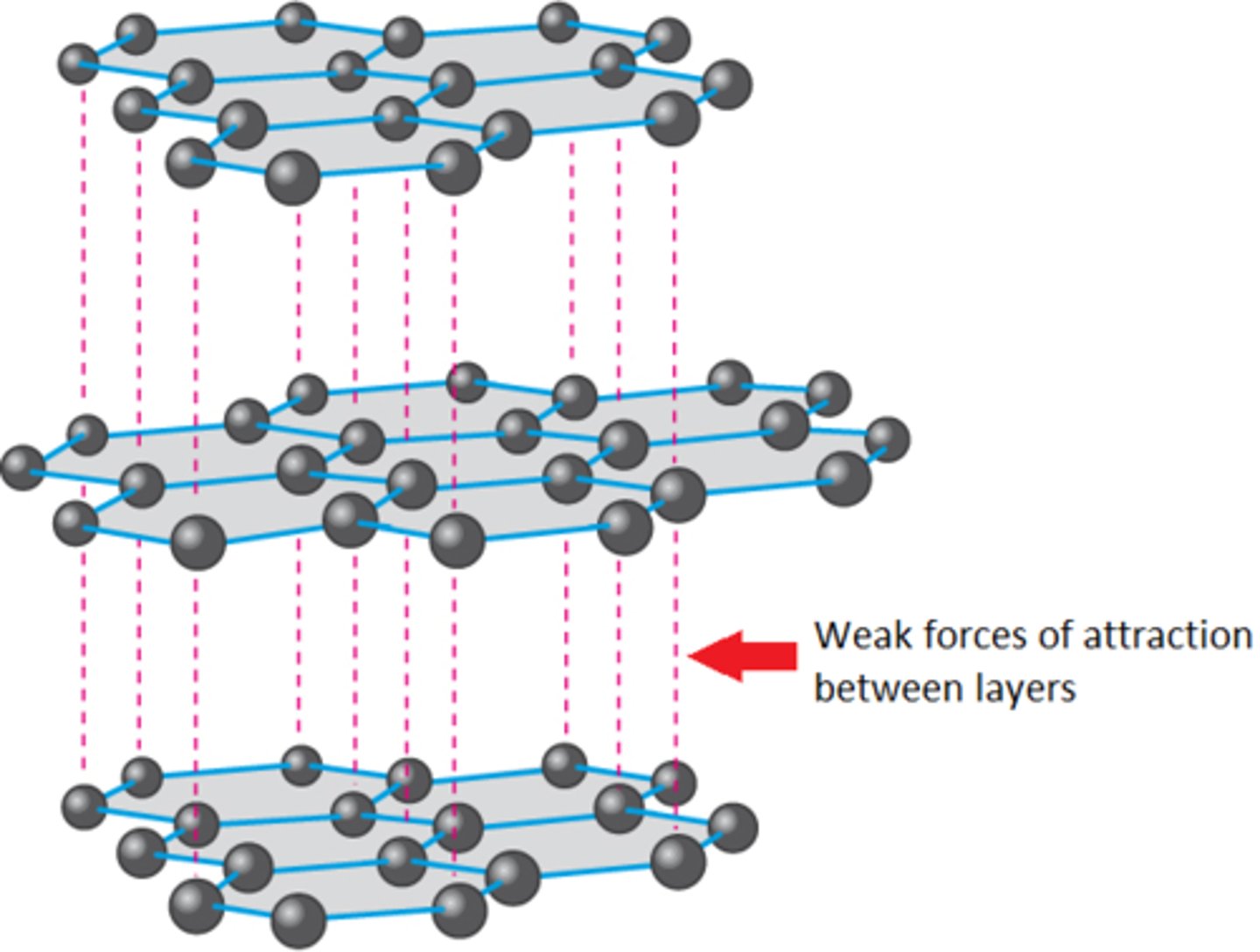
Graphite structure
Fullerene structure