Cell cycle and cell-cell interactions
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
94 Terms
What processes are done during the cell cycle?
genome replication, growth of the cell (to double contents), segregation of chromosomes, and division into two daughter cells
What are the two broad phases of the cell cycle?
Interphase and M phase
What are are the phases within the M phase?
prophase, prometaphase, metaphase, anaphase, and telophase, and cytokinesis.
What phase is longer? Interphase or metaphase
Interphase is usually longer
What are the interphase stages?
G1, S, and G2
What happens in S phase? Where is it in the cycle?
Cells duplicate their DNA and centrosome. IT occurs between G1 and G2.
What is the progression from interphase to M phase?
G1→ S→ G2→ M
What happens during G1 and G2?
The cell grows and enlarges its proteins and organelles to prepare for division.
What does it mean when a cell is 2N?
It means that a cell has 2 copies of each chromosomes, one from mom and one from dad. After S phase a cell is 4N.
What does flow cytometry measure?
DNA contents using fluorescent signal. You can track how much DNA is in the cell.
What does it mean when it is said that the cell cycle is “recursive”?
The cell has to be the same size at the beginning and the end of the cell cycle.
What is the central control system for the cell cycle?
Cyclin-dependent-kinases, or CDKs. They phosphorylate proteins, regulating their behaviors.
What is cyclin? What is its relationship to CDK?
Cyclin is a protein that binds to CDK in order to active it. Make the cyclin-CDK complex. When there is no cyclin present CDK will be inactive.
are cyclin present for the whole cell cycle? are CDKs?
Cyclins are not present for the whole cell cycle, but CDKs are. Cyclin levels are regulated in order to regulate CDK activation.
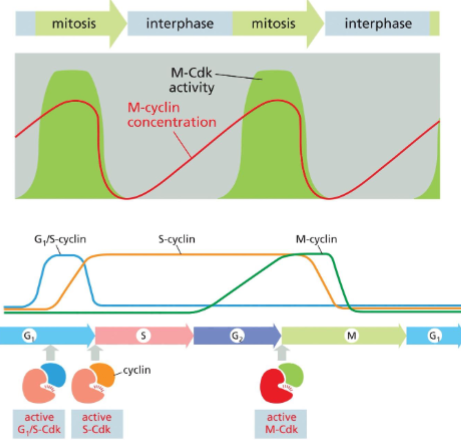
What are M-CDK and M cyclin?
M-CDK is the CDK active during M phase and M cyclin is the cyclin that binds to M-CDK.
How does the presence of M-cyclin fluctuate during the cell cycle?
During interphase, M-cyclin slowly rises as it gets transcribed and translated through the phases and then quickly crashes right at the end of mitosis.
What, other than cyclin, regulates M-CDKs?
Wee1 (kinase) inhibits M-CDK activity and Cdc24 (phosphatase) activates M-CDK.
How come the activation of M-CDK is so rapid?
It is able to phosphorylate the phosphatase Cdc25 and allow it to activate other M-Cyclin. It can also turn off Wee1.
How is cyclin destroyed?
Multiple ubiquitins get covalently bonded to the cyclin forming a chain and targeting the protein for degradation.
What is the proteasome?
A protein that degrades any protein marked with a poly ubiquitin chain.
How do proteasomes degrade proteins?
The chain binds to the structural part of the protease and the protein it’s bound to get unfolded and and woven through the central pore where it is degraded.
What attaches ubiquitin to M-cyclin?
APC/C (APC) a ubiquitin ligase.
How does M-CDK trigger nuclear envelope disassembly?
It phosphorylates nuclear lamins and nuclear pore proteins.
What do G1/S-CDKs do? When are they active?
Promote entry into the cell cycle by activating S-Cdks. They start being activity in the middle of G1 and become inactive at the start of S phase.
When are S-CDKs active? What do they trigger?
They start activity in the G1 phase and are active until the middle of the M phase. They trigger DNA replication.
When are M-Cdks active?
They start activating at the beginning of G2 and become inactive in the middle of M.
What is the different between these two diagrams?
The top graph represents data from early embryo divisions. The bottom diagram represents data from non-embryonic cells.
All cells take the same amount of time to divide? T/F
False, some embryonic cells will divide without growing, making their cell cycles very short. They essentially alternate between G and G2.
How do cells that skip that G1 regulate their M cyclin differently?
The cell doesn’t stop in G1, so there is no need for them to delay M-cyclin synthesis. In a cell that undergoes growth stages, M-cyclin production starts after S phase.
What happens in G1?
Cells grow and perform their normal cellular function. Outside factors decide whether the cell will divide or enter G0.
What are mitogens? What do they do?
They are signaling proteins that bind to a receptor outside of the cell. They activate the synthesis of G1/S -cyclings.
How do mitogens end up trigger the synthesis of S-cyclins?
The G1/S Cdk, activated from the mitogen, phosphorylates Rb (a transcription factor), inactivating it and allowing the cell the transcribe S-cyclin genes.
What are CKIs?
They are CDK inhibitor proteins.
What is p27? What would happen if it were over or under expressed?
It’s the specific CKI that inhibits S-Cdk-Cyclin kinase abilities. If it were not expressed, a cell would probably undergo division too quickly, possibly becoming cancerous. If it were over-expressed, it would probably stop a cell from growing
How is p27 inactivated?
G1/S-Cdk phosphorylates p27 allowing it to get marked for degradation. Once p27 gets degraded, the S-Cdk-Cyclin complex activates.
What causes p27 to get degraded?
Once p27 gets phosphorylated by G1/S CDK, SCF ubiquitinates p27 causing it to be targeted by the proteasome.
SCF only regulates p27 degradation. T/F
False, it regulates G1/S and S cyclin degradation.
What is preRC? When and where does it form?
It is the pre-replicative complex that forms in G1 at the origin of replication. Once S-Cdk is activated it recruits the proteins needed to start replication.
S-Cdk prevents re-replication. T/F
True, S-CDk will phosphorylate some components of the preRC to ensure it will not reload onto the origin.
Why do S-CDK levels stay high after S phase?
S-Cdk is needed to ensure DNA does not get re-replicated.
What are the two things needed for activation of M-Cdk?
M-CDK has to bind to M cyclin and inhibitory phosphates (added by Wee1) have to be removed (by Cdc25).
By the end of M phase all cyclin levels are low due to SCF targeting S and M cyclins for degradation. T/F
False, SCF will target S cyclin for degradation and APC will regulate M cyclins.
What does degradation of cyclins occur so quickly?
To ensure the cell does not slip back in the cell cycle. it ensures the changes are irreversible.
How is cohesin removal and M-cyclin degradation related?
Both require the APC. This system ensure chromosomes separate at the correct time.
How is cohesion removed from chromosomes?
Securin inhibits separase which cleaves cohesin. The APC targets securin for degradation, releasing separase.
What are exit ramps?
Cells not exposed to mitogens can pause or exit the cell cycle. Only present in G1, before a cell is introduced to new mitogens.
Where are the different cell cycle checkpoints?
Before S phase, after G2, and during anaphase.
What does the start checkpoint look for?
It checks for size, available nutrients, and DNA damage (SaND).
How does a cell stop the cell cycle from progressing in G1?
p21 (a CKI) will get transcribed to inactive G1/S and S-Cdks.
What does the G2 checkpoint check for?
Makes sure all DNA is replicated and unbroken.
What will happen if breaks in DNA are detected during G2 check?
G2 checkpoint kinases get activated and inactive Cdc25, halting the cell cycle.
What does the spindle checkpoint monitor?
This checkpoint will check to make sure all chromosomes are properly attached to the spindle.
How does the cell know when to separate chromosomes?
The cell monitors tension at the kinetochores, if one is not under tension, the checkpoint gets triggered.
What monitors tension at kinetochores?
Mad2 proteins. If a KT is unattached, it will have a lot of Mad2. If a KT is attached but not under tension, it will have some Mad2. If a chromosomes is aligned and attached, it will have no Mad 2.
What happens with the spindle checkpoint is activated?
Mad2 will inhibit the APC, stopping both M-cyclin degradation and separase activation. This halts the cell in metaphase.
What happens if a cell can’t correct during a checkpoint halt?
The cell will trigger intrinsic apoptosis.
how do many cancer cell drugs work?
They will target checkpoint activation and other cell cycle machinery to stop over-proliferation of cancer cells.
How did researches find that there is a factor that promotes entry into the M phase?
Researchers fused M and G1 phase cells and found that the G1 cell parts were driven to mitosis. They concluded there must be something in the cytoplasm (M-Cdk -Cyclin) that triggers entry into M phase.
How did researchers find that there are protein (cyclins whose levels fluctuate during the cell cycle?
Researches used sea urchin eggs (whose cell cycles were very quick) to monitor protein levels during the cell cycle. They used gel electrophoresis to analyze proteins present at different cell cycle stages.
How did researched find that Cdks drive the cell cycle?
Researchers used budding yeast and fission yeast to visually identify where a mutated cell would get stuck in the cell cycle. This helped them figure out the proteins needed to drive the cell into different phases.
How did researchers find that the spindle checkpoint gets silenced when chromosomes are under tension?
They found that if they artificially pulled on a chromosome with a microneedle, it would trigger anaphase.
What do you need to build tissue?
interactions between adjacent cells and interaction between the cell and the extracellular matrix (ECM).
What are the two types of interactions cells can have with each other?
Homophilic, mediated by the same protein on each cell. Heterophilic, mediated by different proteins on each cell.
What are epithelial cells?
They are cells that form our skin and line some internal cavities. They have polarity
What is the basal lamina?
It is the ECM.
All junctions are found in non-epithelial cells. T/F
False, all junctions can be found in nonepithelial cells except tights junctions.
What are the major cell-cell junctions?
tight junctions. gap junctions, adheres junctions, and desmosomes.
What are the major cell-EMC junctions?
Hemidesmosomes and focal adhesions
What are tight junctions?
They seal off areas to create barriers, stopping molecules from passing through. They are specific to epithelial cells.
How are cells “sealed” in tight junctions?
Cells are connected by branching strands of claudins and occludins. Kind of like Ziplock bags.
How do proteins polarize epithelial cells?
They can separate protein areas (either the apical or basal side).
What do gap proteins allow through?
Small molecules and metabolites smaller than 1000 daltons.
What are gap junctions comprised of?
They are made up “hemi channels” (connexons) which are made of 6 connexin subunits. Hemichannels from each cell connect to form a pore. This channel can be opened or closed
What do adherens junctions do? How do they manifest in cells
Will link cells and change shape of cells. Allowing cells to make tubes during development.
Adheren junctions are indirectly connected to the actin cytoskeleton. T/F
True. Linker proteins connect adheren junctions to the actin cytoskeleton.
What is the difference between desmosomes and adheren junctions?
Desmosomes link to intermediate filaments instead of actin.
What are cadherin proteins?
A class of cell adhesion proteins. Involved in desmosomes and adheren junctions.
What is the name of the filament in desmosomes connected to linker proteins?
keratin filaments.
How do Hemidesmosomes and desmosomes differ?
Hemidesmosomes link to intermediate filaments in the EMS and not another cell. Integrins, not cadherins, are used to connect.
What are focal adhesions? Why are they important?
They link the EMC to the cell actin - cytoskeleton. It is important in cell movement.
Where do focal adhesions form?
They form at the leading edge of where a cell crawls forward.
What junctions associate with actin?
Adherens and focal adhesions.
What are the 4 types of tissues?
epithelial, connective, muscle, and nervous
Why is the ECM not abundant in epithelial tissue?
Epithelial cells are stabilized by cell-cell interaction between cytoskeleton parts.
Basal lamina is the same from tissue to tissue. T/F
False, basal lamina is muscle tissue surround cells but in epithelial tissue it lines under the epithelial cells.
Integrins can mediate cell-cell and cell-ECM interactions. T/F
True, leukocytes interact with integrins for extravasation and the ECM is connected to the cell in hemidesmosomes and focal adhesions.
What are the components of Integrin? How are they switched on and off?
Made up of an alpha and beta subunit. It folds when it is inactive and extends when active. It can be changed from outer or inner signals.
What are the two types of collagen, how do they differ?
sheet forming collagen and fibrillar collagens. Sheet collagen forms triple helixes and interact at their tail ends, creating a branched network through out the ECM. Fibrillar collagen, makes fibers like steel fibers in a bridge.
What is special about how fibrillar collagen is processed?
Collagen is secreted from a cell with domains that make it unable to form fibers. In the ECM a protease is able to cleave those domains.
What is the purpose of proteoglycans? How?
They allow the cell to have a gel like consistency due to their long sugar chain making their difficult to pack.
What are multi adhesive matrix proteins?
They are involved in linking all the components of the ECM. It also helps with cell-ECM interactions
What is a laminin?
It is a cross shaped component of the basal lamina, and it allows integrin to bind to ECM components. i
What do Fibronectin connect?
It can connect both kinds of collagen and integrin, linking cells to ECM in a very stable way.
When does loose adhesion or tight adhesion get used in extravasation?
Loos adhesion is used when the cell rolls and sugar residues interact weakly. Tight adhesion happens between integrins and leukocytes when the cell needs to slip through the epithelial cells.