Physics II Lesson 12: Thermodynamics Part II
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
True or False? The Second Law of Thermodynamics states that Heat can flow from cold to hot as long as the overall heat flow is from hot to cold.
False. The Second Law of Thermodynamics states that Heat will never be seen to flow from cold to hot.
Compare the term microstate and macrostate?
A microstate is an exact arrangement/state of particles. There are often countless possible arrangements.
A macrostate is a generic state of particles. For instance, "mixed up" vs. "separated" are examples.
Why doesn't heat ever flow from cold to hot?
Because statistically speaking there are way more microstates in which the fast moving particles and slow moving particles are mixed up.
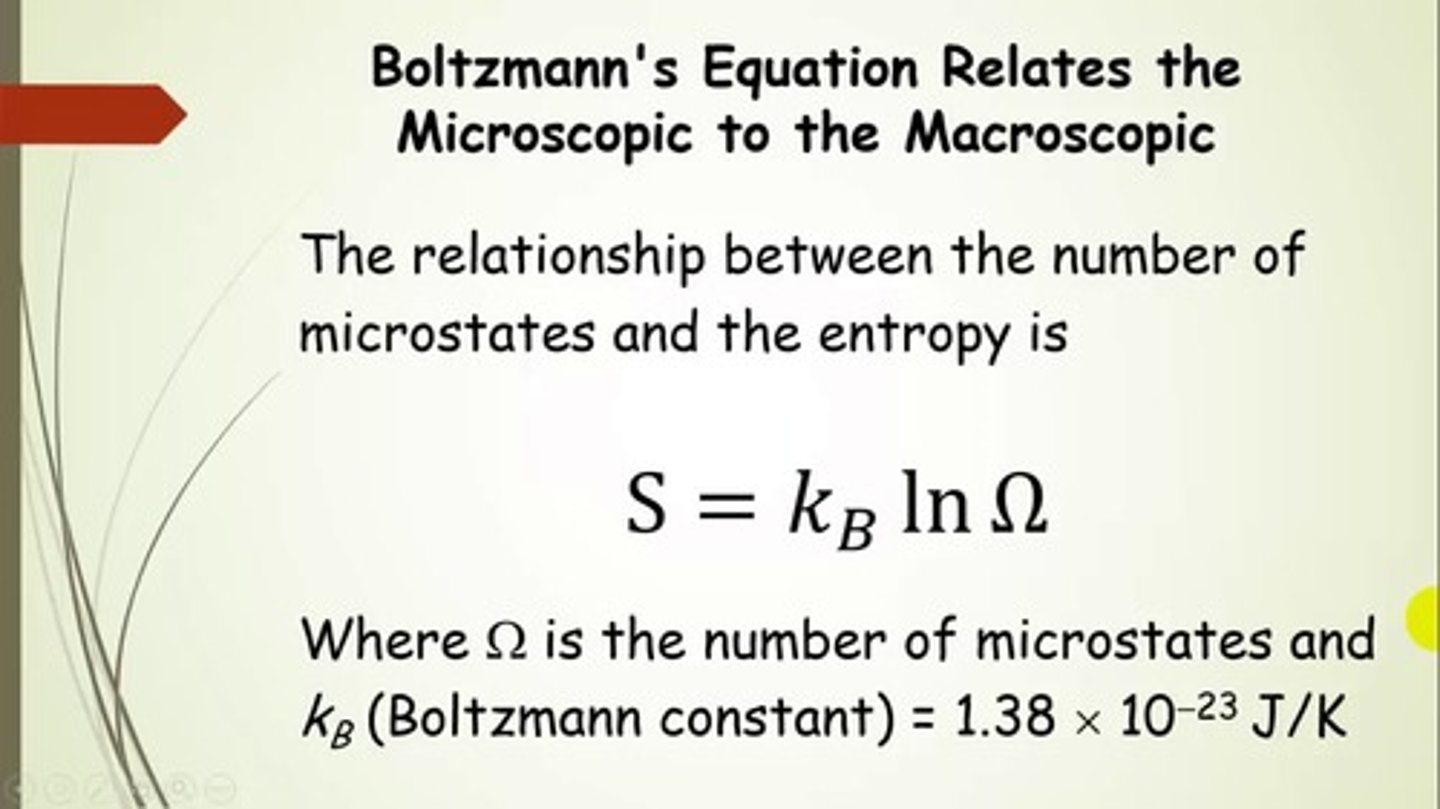
What equation can be used to relate Entropy to the number of possible microstates?
CRB True or false? It is better to think of Entropy as energy spontaneously spreading out when possible, rather than things being disordered.
It is better to think of Entropy as energy spontaneously spreading out when possible, rather than things being disordered.
CRB Which of the following can Entropy measure?
(A) How much energy is spread out.
(B) How widely energy spreads out during some process
(C) Both A and B
(D) Neither A nor B
(C) Both A and B
Entropy can measure each of the following:
(A) How much energy is spread out.
(B) How widely energy spreads out during some process
CRB True or false? Although the universe is a closed system, it is always expanding, so entropy can continue to increase.
True. Although the universe is a closed system, it is always expanding, so entropy can continue to increase.
CRB In which of the following types of systems would any thermodynamic process have to either increase entropy or keep entropy the same?
(A) Open System
(B) Closed System
(C) Both A and B
(D) Neither A nor B
(B) Closed System
In a Closed system, any thermodynamic process must keep entropy the same or increase it.
CRB In regards to comparing processes, compare Natural and Unnatural Processes.
Natural Processes are the Irreversible processes that you expect to happen, like two objects in contact reaching thermodynamic equilibrium.
Unnatural Processes would be things that would not normally occur, like ice becoming even colder if left at room temperature.
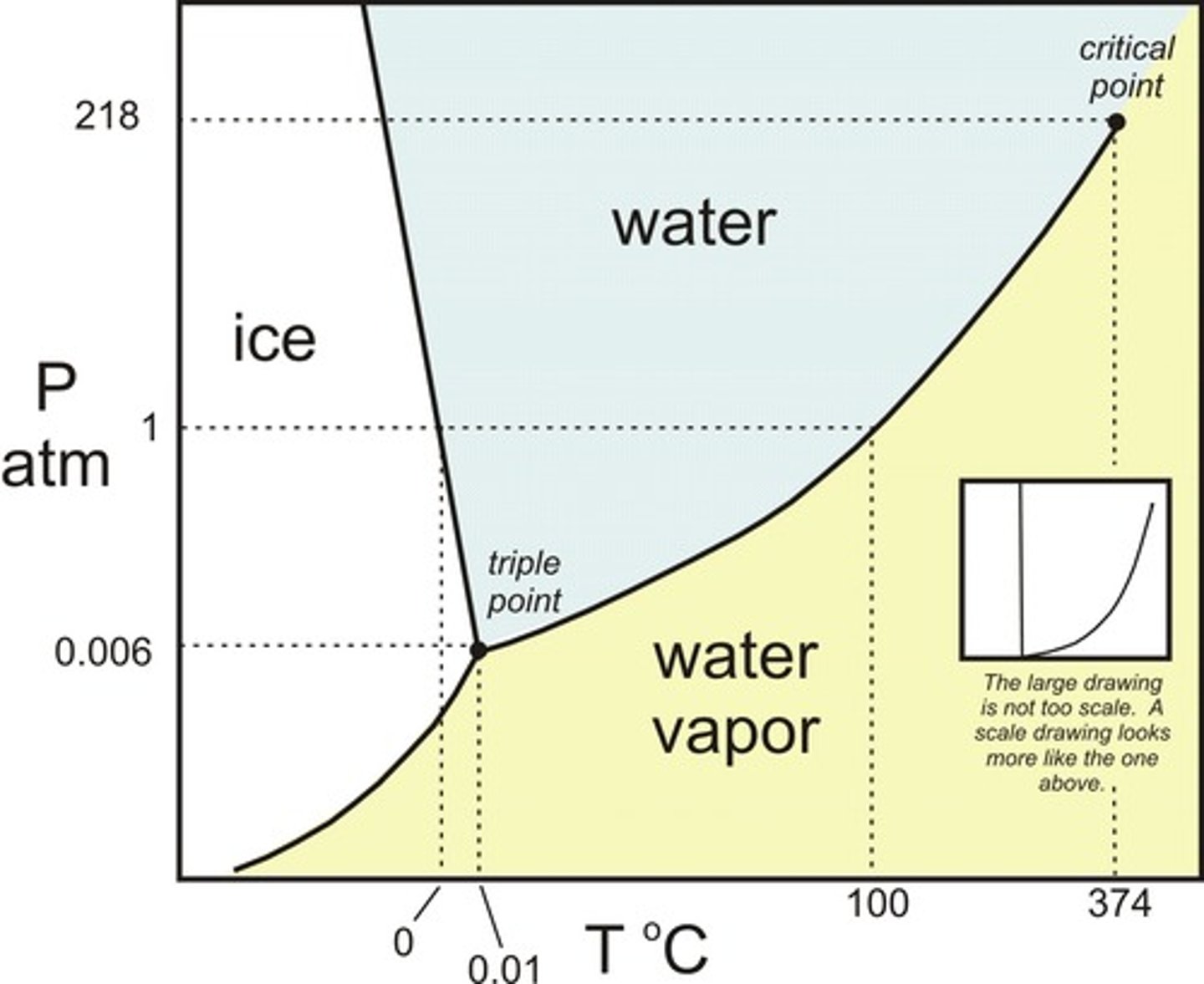
Draw the Phase Diagram for water. Be sure to label the x- and y-axes. Also, label the Critical Point as well as the Triple Point.
CRB True or false? The number of microstates in solids cannot change until the solid starts to melt.
False. The number of microstates in solids will increase as the temperature of the solid increases.
Water will boil at a __________ temperature on the top of Mount Everest than at sea level. Water will freeze at a __________ temperature on the top of Mount Everest than at sea level.
(A) higher, higher
(B) higher, lower
(C) lower, lower
(D) lower, higher
(D) lower, higher
Water will boil at a lower temperature on the top of Mount Everest than at sea level. Water will freeze at a higher temperature on the top of Mount Everest than at sea level. This can be determined using the previous Phase Diagram for water.
CRB True or false? Because evaporation takes some of the highest-energy liquid molecules and makes them enter the gas phase, evaporation is an exothermic process.
False. Because evaporation takes some of the highest-energy liquid molecules and makes them enter the gas phase, evaporation is an Endothermic Process, with the liquid as the heat source.
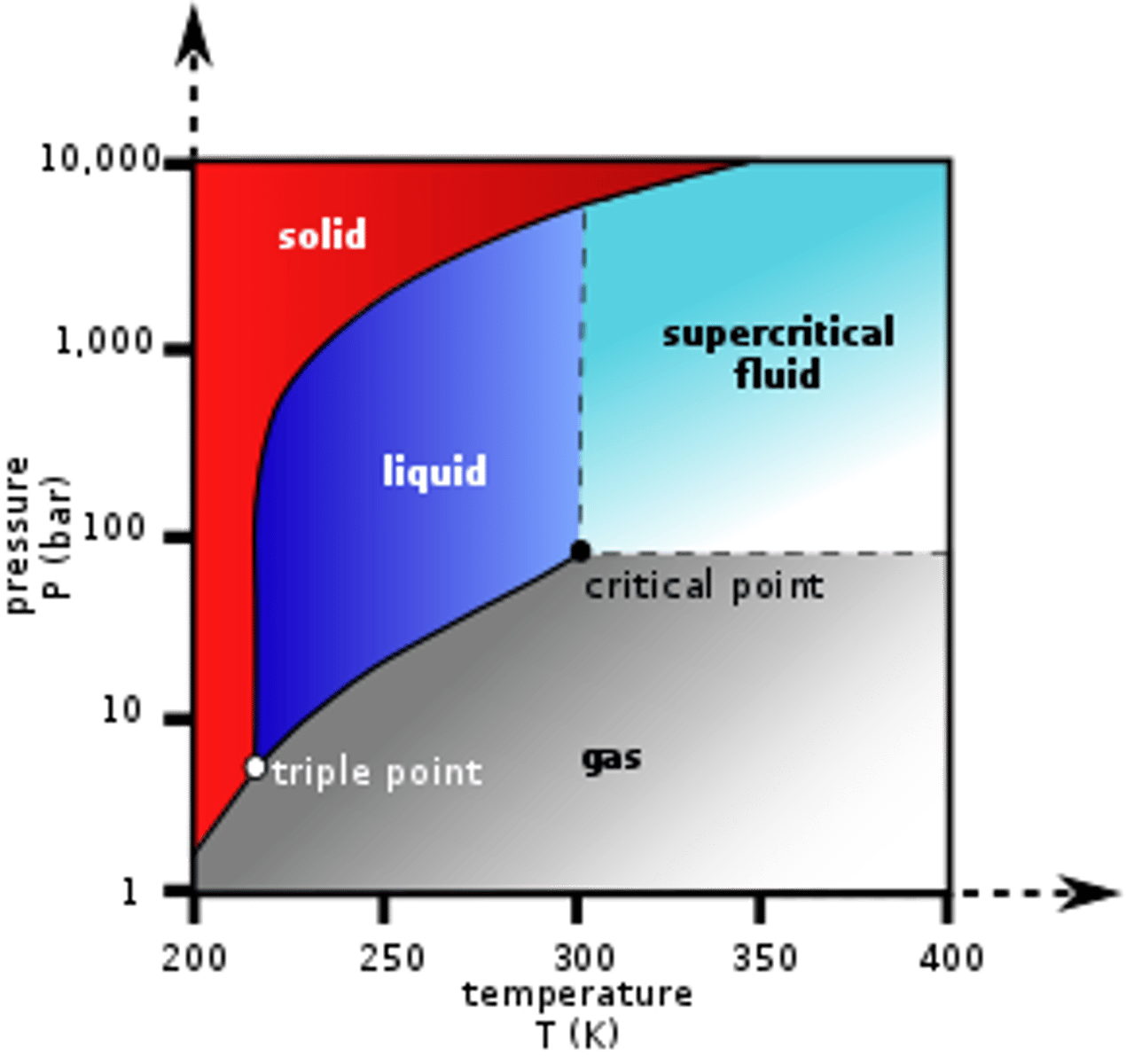
Draw the Phase Diagram for Carbon Dioxide. Be sure to label the x- and y-axes. Also, label the Critical Point as well as the Triple Point.
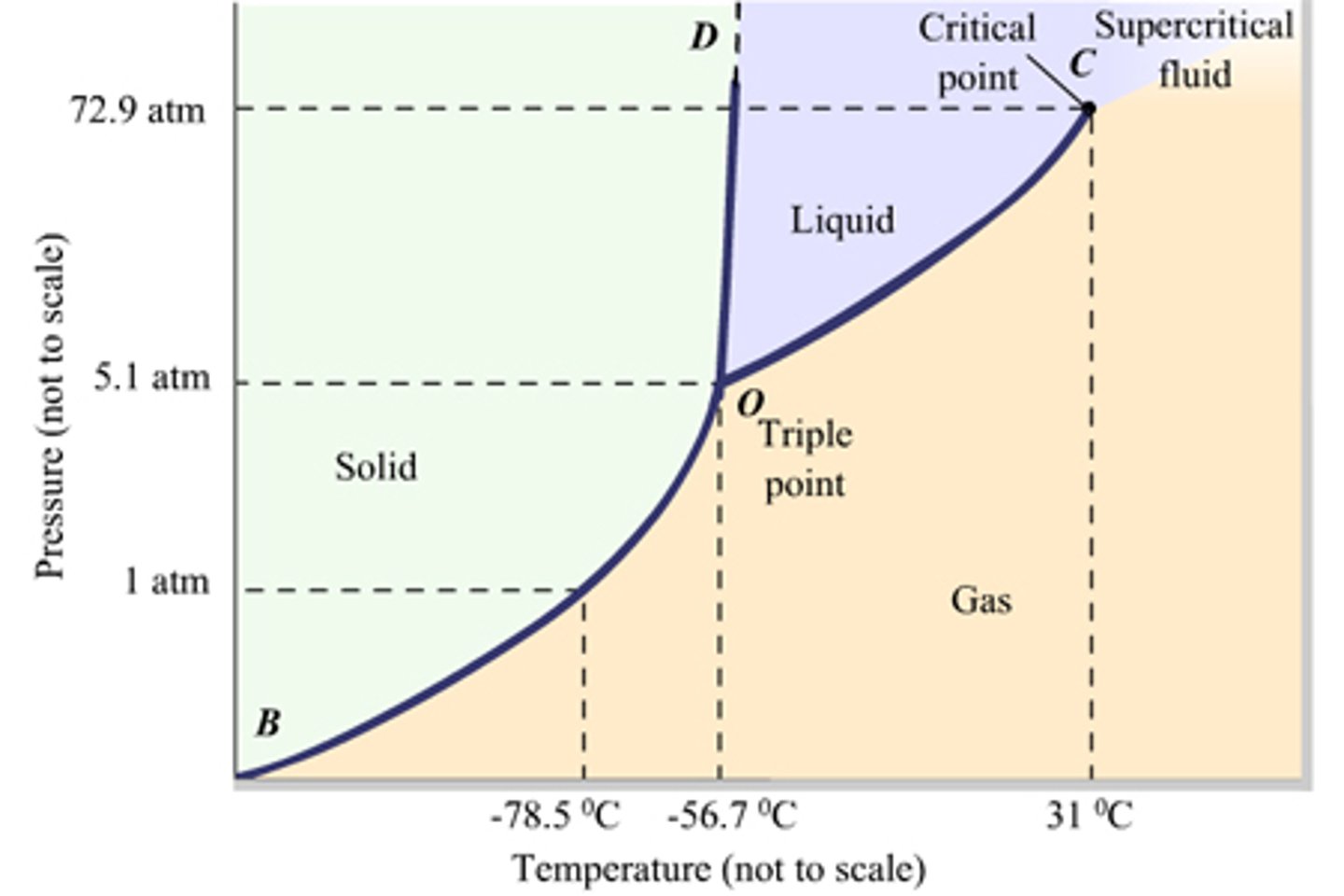
CRB Fill in the blanks: At very low pressures and temperatures, a solid may transition to a gas in a process called _______________; the reverse process is called ______________.
(A) Melting, Freezing
(B) Vaporizing, Condensing
(C) Sublimation, Deposition
(D) Supercritical Evaporation, Supercritical Freezing
(C) Sublimation, Deposition
At very low pressures and temperatures, a solid may transition to a gas in a process called Sublimation; the reverse process is called Deposition.
CRB True or false? The specific heat of a substance depends upon the phase the substance is in.
True. The specific heat of a substance depends upon the phase the substance is in.
Above the Critical Point, the molecules are considered a Supercritical Fluid. What is that?
A Supercritical Fluid is a fluid with both liquid- and gas-like properties.
How does Enthalpy (∆H) relate to Q?
Enthalpy (∆H) = Q
What equation can be used to calculate the Enthalpy change for a reaction given Heat of Formation values?
∆H = Hf(products) - Hf(reactants)
Consider the following unbalanced reaction:
C6H12O6(s) + O2(g) -> CO2(g) + H2O(l)
What is the enthalpy change (in kJ) for the combustion of 2 mols of glucose considering the following Heat of Formation values:
C6H12O6 = -1260 kJ/mol
CO2 = -393.5 kJ/mol
H2O = -285.8 kJ/mol
(A) -1,292
(B) -5631
(C) 646.2
(D) 5631
(B) -5631
First, balance the reaction as follows:
C6H12O6(s) + 6O2(g) -> 6CO2(g) + 6H2O(l)
∆H = Hf(products) - Hf(reactants)
∆H = [6(-393.5)+6(-285.8)] - (-1260)
∆H = approx. -3000 (actual: -2816) for one mole of glucose
∆H = approx. -6000 kJ (actual: -5631)