Organic Chemistry Ch. 9 - Alkyne Reactions
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
type of rxn forming alkynes
E2 from dihalide
geminal dihalide
halides on same carbon
vicinil
halides on adjacent Cs
reagents for alkynide ion formation
NaNH2 & NH3
reagents to protonate alkynide ion
excess NaNH2, NH3 & H2O
rxn to reduce
catalytic hydrogenation
reagents to fully reduce alkynes via catalytic hydrogenation
H2
Pt/Pd/Ni
reagents to isolate intermediate in reduction of alkyne via catalytic hydrogenation - syn/cis
Lindlar’s catalyst, P-2 (Ni2B)
reagents to isolate intermediate in reduction of alkyne via catalytic hydrogenation - anti/trans
Na
NH3
reagents alkyne hydrohalogenation markovnikov
H-X
reagents alkyne hydrohalogenation antimarkovnikov
H-X
ROOR
when does alkyne hydrohalogenation form geminal dihalide
excess reagents used (rxn occurs 2X)
alkyne hydrohalogenation is reverse of…
dihalide eliminations
reagents acid-catalyzed hydration of alkynes markovnikov
H2SO4 & H2O
NaOH
similar to oxymercuration - demercuration
product of acid-catalyzed hydration of terminal alkynes markovnikov
enol that tautomerizes into methyl ketone via acid-catalyzed keto-enol tautomerization
reagents acid-catalyzed hydration of alkynes antimarkovnikov
R2BH or H2O2 &NaOH
product of acid-catalyzed hydration of terminal alkynes antimarkovnikov
aldehyde
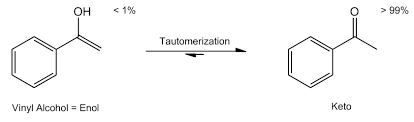
tautomerization
rapid interconversion of 2 constitutional isomers (tautomers) via proton transfers
what acid-catalyzed keto-enol tautomerization looks like
ok it’s not showing up but know this
hydroboration-oxidation of alkynes reagents
R2BH (dialkylborane)
hydroboration-oxidation of alkynes markovnikov or antimarkovnikov
anti-markovnikov
product of hydroboration-oxidation of alkynes
enol that tautomerizes into ketone via base-catalyzed keto-enol tautomerization
reagents for halogenation of alkynes dihalide
one equivalent X2
CCl4
reagents for halogenation of alkynes tetrahalide
excess (2X+) X2
CCl4
halogenation of alkynes stereoselectivity
mainly anti, some syn
product of ozonolysis of internal alkynes
2 carboxylic acids
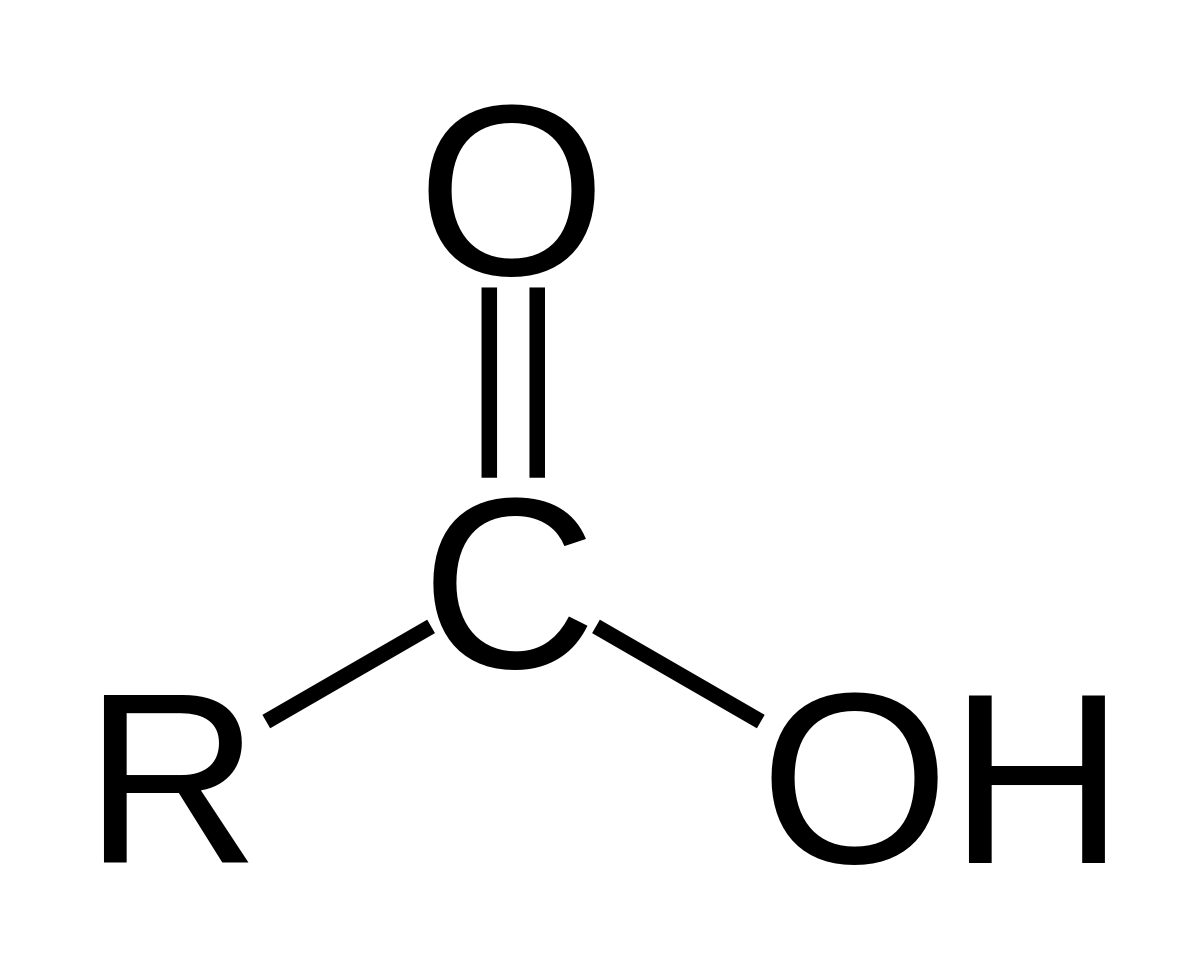
product of ozonolysis of terminal alkynes
carboxylic acid & CO2
reagents ozonolysis of alkynes
O3
H2O
largest difference between alkynes and alkenes
alkylation
alkylation
can change the number of C-C bonds and add/change functional groups, terminal alkynes easily deprotonated to alkynide ions which are strong nucleophiles & bases
alkylation of acetylene
2 successive alkylations
reagents for alkylation
1) NaNH2
2) RX
general synthesis strategy alkene to alkyne
1) halogenation 2) elimination