Acid Base Disorders I
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
What are the first and second line defenses against pH changes?
1) Chemical Buffers are the first line defense against pH changes, and are immediate
→ Bicarbonate, Phosphate, Protein
2) Physiologic Buffers are the second line of defense and vary in the time it takes for them to become active
→ Respiratory Mechanisms work in minutes to hours
→ Renal Mechanisms work in hours to days
What is the role of the lung and kidneys in acid-base regulation?
Lung
→ regulates pCO2 by blowing off carbon dioxide production with alveolar ventilation
→ CO2 is considered a volatile acid
Kidneys
→ regulates bicarbonate levels by reabsorbing filtered bicarbonate as well as replacing bicarbonate lost in our body’s buffering systems (urea) in the proximal tubule
→ bicarbonate is regenerated via distal tubule hydrogen ion secretion and ammonium generation
→ also aids in excretion of fixed acids
What stimulates and inhibits proximal bicarbonate reabsorption?
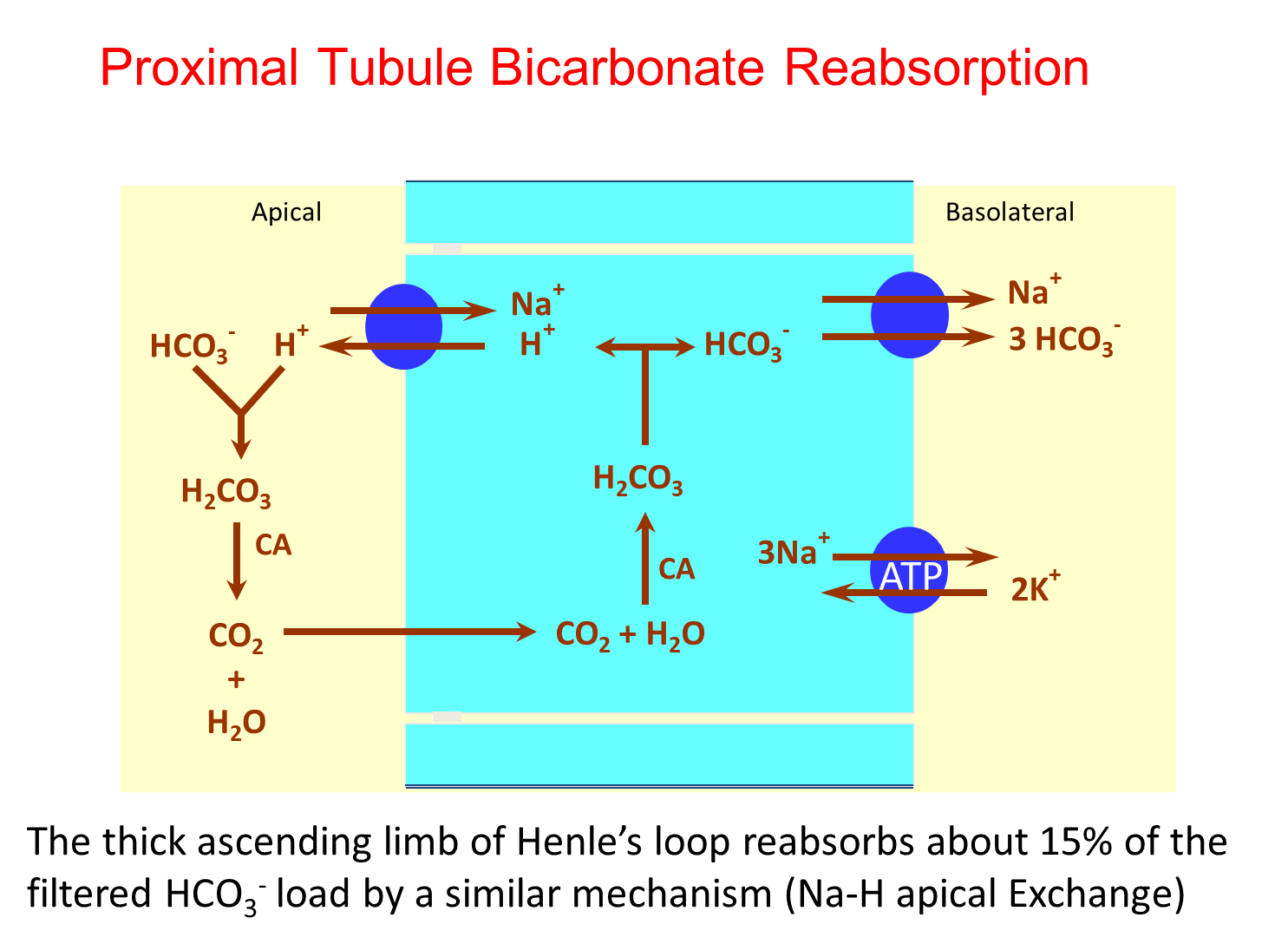
Bicarbonate reabsorption in the proximal tubule occurs via conversion of filtered bicarbonate into CO2 and Water via carbonic anhydrase IV
→ will reform intracellularly via the action of carbonic anhydrase II
1) Stimulated
→ volume depletion
→ chloride depletion
→ acidemia
→ hypokalemia
→ hypercapnia
2) Inhibited
→ Volume expansion
→ alkalemia
→ hyperkalemia
→ hypocapnia
What are the two methods of acid excretion?
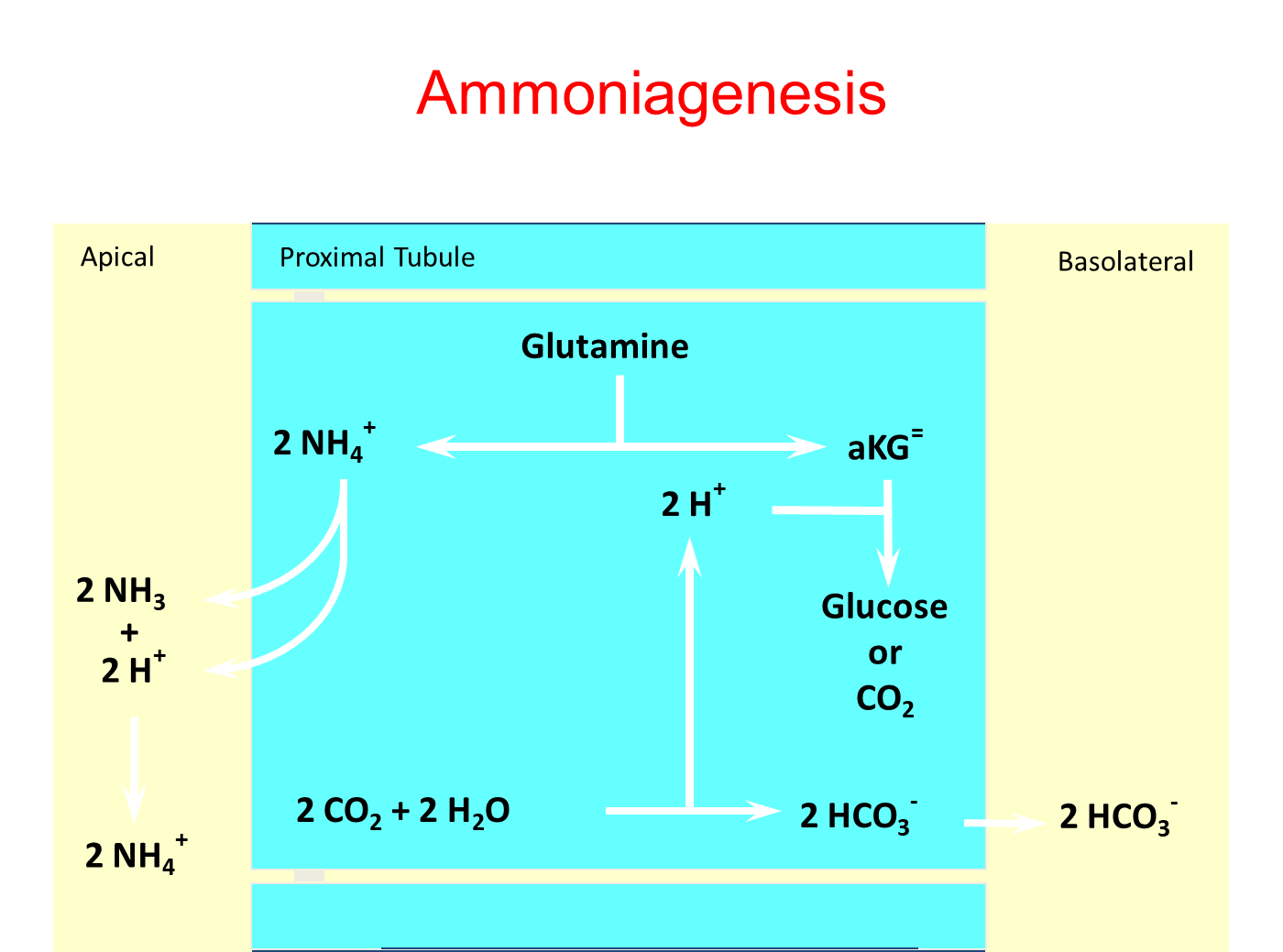
Titratable Acid Excretion
→ protons secreted into the nephron are buffered by things like phosphate and excreted
Ammonium Generation
→ ammonium formed and secretion into the proximal tubule which is then reabsorbed at the thick ascending loop
→ becomes ammonia and is then reformed into ammonium and secreted into the collecting tubule (ammonium trapping)
→ this process is stimulated by acidemia and hypokalemia and is inhibited by the opposite
What stimulates and inhibits distal hydrogen secretion?
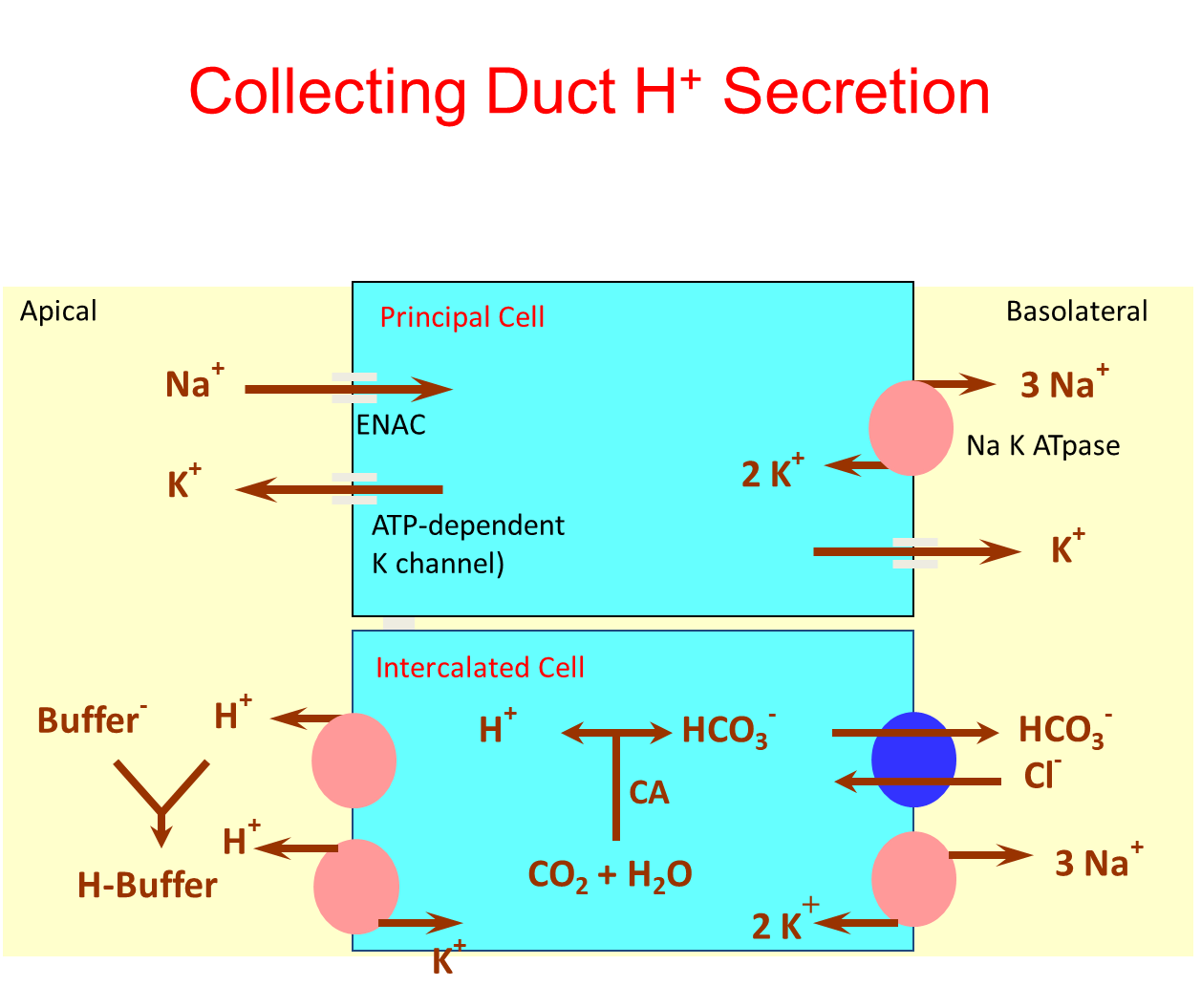
Secretion at the distal tubule of hydrogen will favor bicarbonate generation and absorption into the interstitium
→ this occurs because carbonic anhydrase combining CO2 and water will leave us with a formed bicarbonate and hydrogen that will move in opposite directions
1) Stimulates
→ increased distal sodium delivery - activation of ENaC will lead to increased potassium and hydrogen secretion
→ Excess mineralocorticoid effect
2) Inhibitory
→ Decreased distal sodium delivery
→ hypoaldosteronism
→ urinary buffer deficiency
What is the difference between Acidosis, Alkalosis, Acidemia, and Alkalemia?
Acidosis - process that decreases blood pH
Alkalosis - process that increases blood pH
Acidemia - arterial blood pH < 7.36
Alkalemia - arterial blood pH > 7.44
What is the difference between a simple disturbance and a mixed disturbance?
Simple Disturbances
→ single acid base disorder and expected compensation occurs - respiratory/metabolic acidosis and alkalosis
→ if there is an issue with the lungs there will be an issue with the kidneys, and vice versa
Mixed Disturbances
→ two or more primary acid base disturbances are present, having two disturbances at the same time
→ arterial blood pH will depend on the direction and magnitude of the disturbances
What is the difference between anion gap and non-anion gap acidosis?
Anion Gap Formula = [Na+] - ([Cl-] + [HCO3-])
with left representing unmeasured anions while the right side of the equation representing unmeasured cations
1) Anion Gap
→ anion gap acidosis occurs when the anion gap is elevated usually greater than 12
→ caused by conditions that add acid to the blood that are not chloride or bicarbonate, leading to an increase in gap
2) Non-Anion Gap
→ acidosis with a normal anion gap (in between 8-12)
→ acid production in the body is normal, but there is either impaired acid excretion, increased bicarbonate loss, or a increase in hydrogen ion gain
What is the mnemonic for Anion Gap Acidosis?
GOLDMARK
Glycols
Oxoproline
Lactic Acid
D-Lactate
Methanol
Aspirin
Renal Failure
Ketoacidosis (starvation, diabetic, alcoholic)
What is Osmolar Gap?
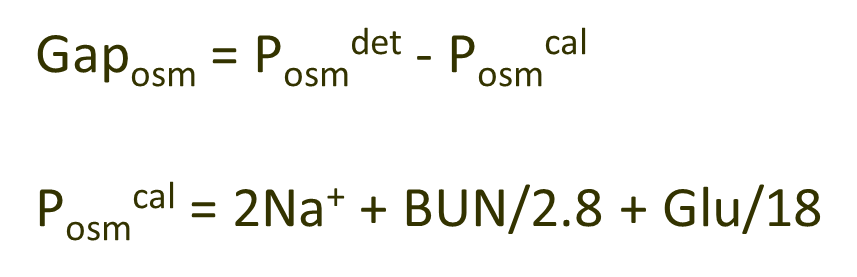
Osmolar Gap is the difference between the measured serum osmolality and the calculated serum osmolality
→ a gap greater than 10 mOsm/kg there is a possible toxin ingestion suggestive of methylene alcohol and ethylene glycol ingestion or ketosis
What findings would be consistent with excessive methanol, ethylene glycol, and alcoholic ketoacidosis
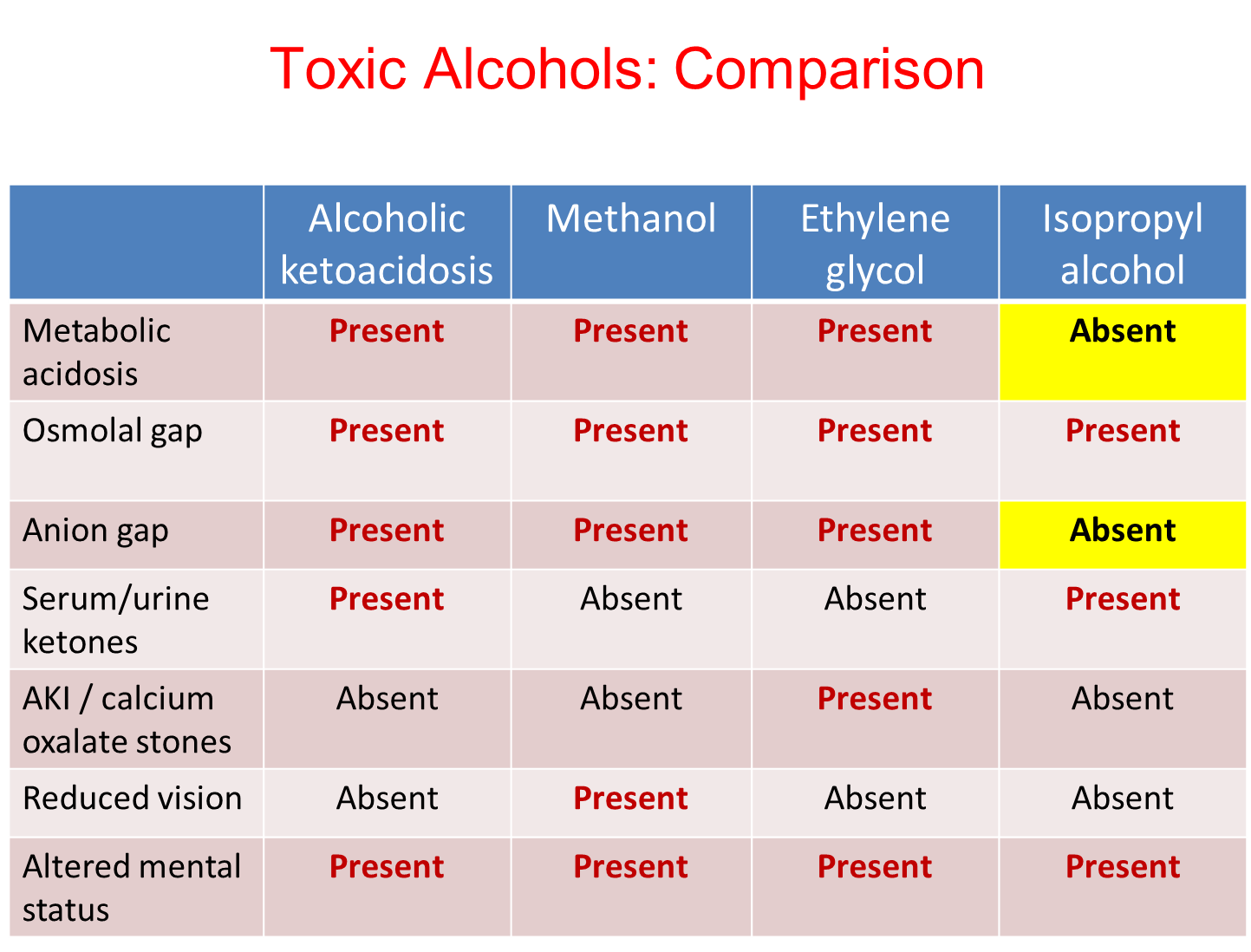
All three are forms will be metabolic acidosis with both an anion and osmolar gap. All patients will also have a altered mental status
1) Alcoholic Ketoacidosis
→ Serum Urine Ketones will be present
2) Methanol
→ Reduced vision
3) Ethylene Glycol
→ Acute Kidney Injury or the presence of calcium oxalate stones
What are the causes of non-anion gap acidosis?
Non Anion Gap Metabolic Acidosis is caused by either issues with kidneys or the gut
1) Impaired Renal Excretion
→ Renal Failure
→ Classic Distal Renal Tubular Acidosis (Type I)
→ Hyperkalemic Distal Renal Tubular Acidosis (Type IV)
2) Renal Bicarbonate Loss
→ Proximal Renal Tubular Acidosis (Type II)
→ Therapy of diabetic ketoacidosis
3) Acid Gain
→ Hyperalimentation of Fluids
→ Ammonium Chloride Ingestion
4) Gastrointestinal Bicarbonate Loss
→ Diarrhea
→ Pancreatic Drainage
→ Ureteral Diversion