isotopes/rfm/moles
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
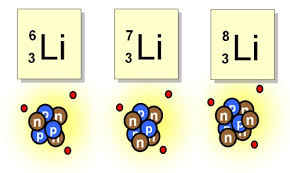
what are isotopes
isotopes are atoms of the same element but with a different number of neutrons. so two elements but with different mass numbers this is because of the different amount of neutrons
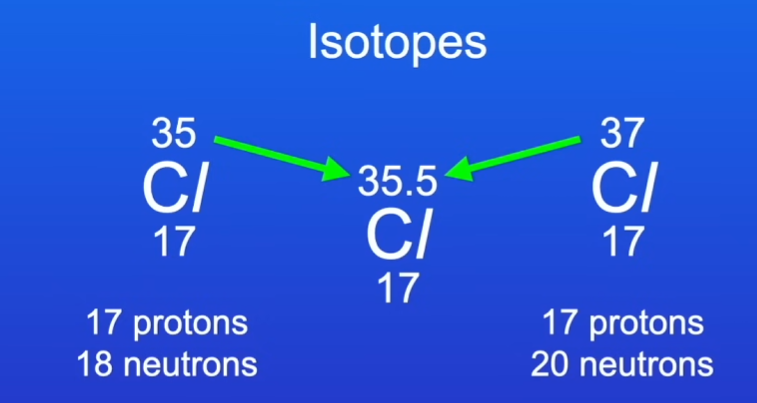
what is relative atomic mass ar
the relative atomic mass is the weighted average of an atom of an element so if there are multiple isotopes with different mass numbers they are weighted into a average and this is called the relative atomic mass
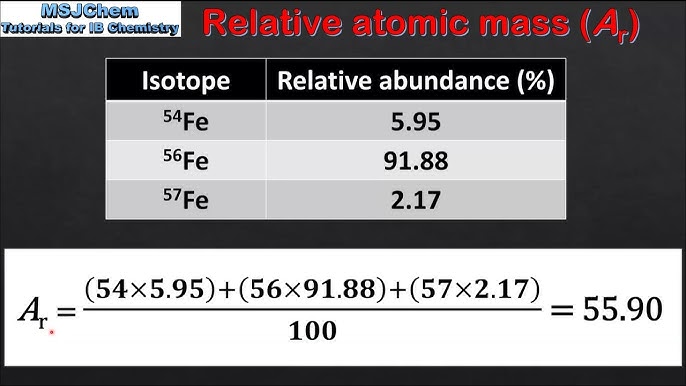
what is the formula for relative atomic mass
so to calculate relative atomic mass you have to get the abundance of the first isotope and multiply its mass number then do the same with the next isotope and put the two calculations for each isotope in brackets and then + and then divide this by 100 Ar = Σ (Isotopic Mass × % Abundance) / 100
what is relative formula mass Mr
relative formula mass is adding all of the relative atomic masses so example calculate relative formula mass of h20
h=1 o=16 so there are two oxygens and 1 hydrogen so add up 1+16+16 to get the relative formula mass Mr relative formula mass also has no units
calculate the relative formula mass of Cu(No3)2
so Ar of Cu is 63.5 Ar of N is 14 and Ar of O is 16 so
29+7×2+16×6=187.5
what is a mole
a mole is the amount of a substance that contains 6.02×10²³
what is the formula for counting how many moles there are in a substance
amount(mol)=mass(g)/molar mass(gmol-1)
calculate amount of moles in KMNo4 in 1.58g
calculating molar mass is adding up the relative atomic masses of each element in that substance so K=39.1 Mn=54.9 and O=15×4 =158 so that’s the molar mass
amount=1.58/158=0.0100mol
how to calculate the amount of moles in a solutions
moles=concentration x volume dm³/1000
the reason for the /1000 is because usually you have to convert the units yourself so 10cm³ in dm³ is 10/1000=0.01dm³
what is concentration
concentration just tells you how much solute is dissolved in a volume of solution and basically used to tell you how strong or dilute a solution is
it is measured in mol/dm³
calculate the amount of NaCL in a solution with a volume of 250cm³ and a concentration of 0.100mol
amount=conc mol/dm³ x volume dm³
0.100 × 250cm³/1000 =0.025mol