CHEM 1211 Sections 8.3-8.4 (Electrolytes and Nonelectrolytes, Acid-Base Reactions)
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Electrolytes
substances that dissolve in water to produce a conductive solution
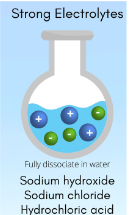
Strong Electrolytes
electrolytes that dissociate completely
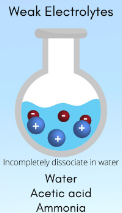
Weak Electrolytes
electrolytes that dissociate only a little
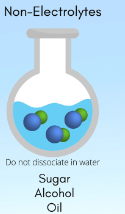
Nonelectrolyte
electrolytes that do not dissociate
Strong Acid
ionizes nearly 100% in water; forms strong electrolytes
Weak Acid
ionizes partially in water (usually less than 10%); forms weak electrolytes
WEAK!!!!!
There are only 6 common strong acids. All other acids are ____
Strong Base
ionic compounds containing hydroxide ions; dissociate 100% in water; forms strong electrolytes
Weak Base
molecular compounds such as ammonia; reacts with water to a small extent and produces hydroxide ions in water
soluble
Only _______ hydroxide compounds are strong bases.
Amphirotic
a substance that can function as an acid OR a base