Chap 10B - Organic chemistry
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
Describe purpose of hybridisation
If carbon were to use its 2s and 2p orbitals for bond formation, many structural features of carbon compounds cannot be accounted for
For example, in CH4, the bond angle would be 90° instead of the actual value of 109.5° and the four C-H bonds
Theory of hybridisation (mixing of orbitals) was proposed to address these discrepancies
Describe characteristics of hybridised orbitals
Each sp, sp2 or sp3 hybrid orbital has one lobe larger than the other
In bond formation, the larger lobe is used -> more effective overlapping -> stronger bonds than if the unhybridised 2s or 2p orbitals had been used
The more s character a hybrid orbital has, the closer the electrons are to the nucleus
More s character -> stronger bond
There is greater s-character in the sp2 hybrid orbital than in the sp3 hybrid orbital and the electrons in the sp2 hybrid orbital are closer to the nucleus -> C-C bond with sp2-sp2 overlap is shorter than that with sp3-sp2 overlap
Describe sp3 hybridisation
Four orbitals of carbon (2s, 2px, 2py, 2pz) are 'mixed' to form four equivalent sp3 hybrid orbitals which are tetrahedral and at 109.5° to one another
The hybrid orbitals have the same energy
The carbon atom uses the four sp3 hybrid orbitals to form 4 sigma bonds (all single bonds) with other atoms
Each sp hybrid orbital contains ¼ s and ¾ p character
Eg. Trigonal pyramidal: 4 regions of electron densities (3 sigma, 1 lone) = sp3 hybridised
Describe sp2 hybridisation
Three orbitals of carbon (2s, 2px, 2py) are 'mixed' to form three equivalent sp2 hybrid orbitals which are trigonal planar and at 120° to one another.
One 2pz orbital of carbon remains unchanged
The carbon atom uses the three sp2 hybrid orbitals to form 3 sigma bonds and the unhybridised 2p orbital to form 1 pi bond (1 double bond present) with other atoms
Each sp2 hybrid orbital contains ⅓s and ⅔ p character
Describe sp hybridisation
2 orbitals of carbon (2s, 2px) are ‘mixed’ to form 2 equivalent sp hybrid orbitals which are linear and 180 degrees to each other
2 2p orbitals of carbon remain unchanged
Carbon atom uses 2 sp orbitals to form 2 sigma bonds and 2 unhybridised 2p orbitals to form 2 pi bonds (triple bond or 2 double bonds) with other atoms
Each sp hybrid orbital contains ½ s and ½ p characters
Describe hybridisation in CH4
Carbon atoms forms four sigma bonds:
Number of hybrid orbitals: 4
Type of hybridisation: sp3
Shape: tetrahedral around carbon atom, bond angle = 109.5°
Sigma bond formation: each C-H bond is formed by the head-on overlap of sp3 hybrid orbital of C with 1s orbital of H
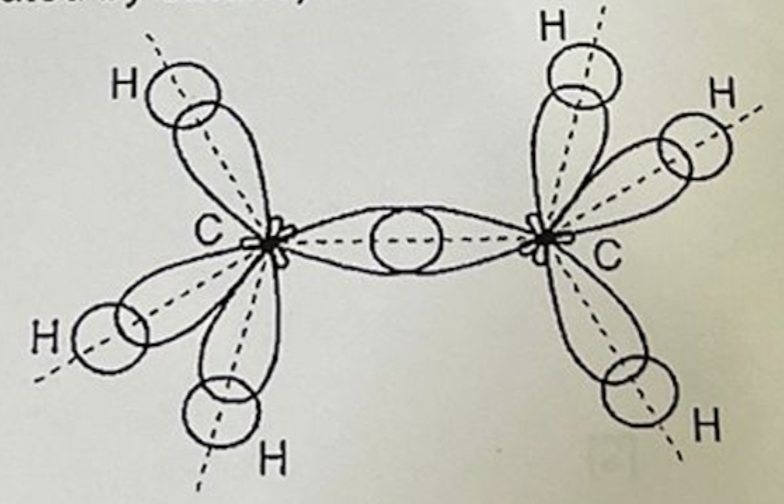
Describe hybridisation in C2H6 ethane
Each carbon atom forms four sigma bonds:
Number of hybrid orbitals: 4
Type of hybridisation: sp3
Shape: tetrahedral around each carbon atom, bond angle = 109.5°
Sigma bond formation: each C-H bond is formed by the head-on overlap of sp3 hybrid orbital of C with 1s orbital of H and the C-C bond is formed by the head-on overlap of sp3 hybrid orbital of each C
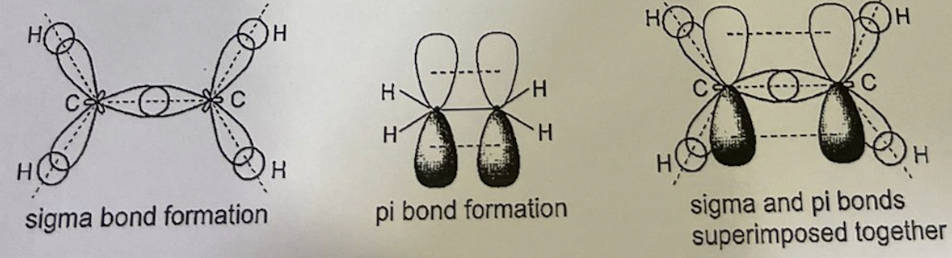
Describe hybridisation in ethene C2H4
Each carbon atom forms three sigma bonds and one pi bond:
Number of hybrid orbitals: 3
Number of p orbitals: 1
Type of hybridisation: sp2
Shape: trigonal planar around each carbon atom, bond angle = 120°
Sigma bond formation: each C-H bond is formed by the head-on overlap of sp2 hybrid orbital of C with 1st of H and the C-C bond is formed by head-on overlap of sp2 hybrid orbital of each C
Pi bond formation: carbon-carbon pi bond is formed by the side-on overlap of 2pz orbital of each C laterally -> resulting pi electron cloud is distributed above and below the plane or atoms
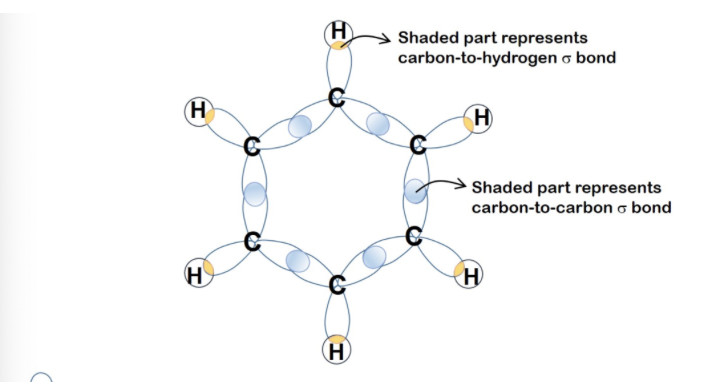
Describe hybridisation in Benzene, C6H6
Each carbon atom forms three sigma bonds and one pi bond:
Number of hybrid orbitals: 3
Number of p orbitals: 1
Type of hybridisation: sp2
Shape: trigonal planar around each carbon atom, bond angle = 120°
Hence, benzene has a planar structure in which the six carbon atoms are bonded in a regular hexagonal ring
Sigma bond formation: each C-H bond is formed by head-on overlap of sp2 hybrid orbital of C with 1s orbital of H and the C-C bond is formed by the head-on overlap of sp2 hybrid orbital of each C
Pi bond formation: C-C pi bond is formed by the side-on overlap of 2pz orbital of each C with the adjacent 2pz orbitals on either side of it
Describe electron cloud in benzene
Since these six 2p orbitals are parallel to one another, each of the 2p orbital can overlap sideways and equally with the adjacent two 2p orbitals
Each of the six 2p unhybridised orbital contains an electron
Hence, the sideways overlap of the 2p orbitals results in a pielectron cloud above and below the hexagonal plane of carbon atoms
NOTE:
The pi electrons move over a larger region of space in the actual structure benzene, as compared to the Kekule structure
The pi electrons are delocalised around the whole ring (resonance)
Each C-C bond in benzene is a partial double bond and is identical to one another
Structure of benzene is more accurately presented as (somewhere above) where the circle represents the delocalisation of six pi electrons
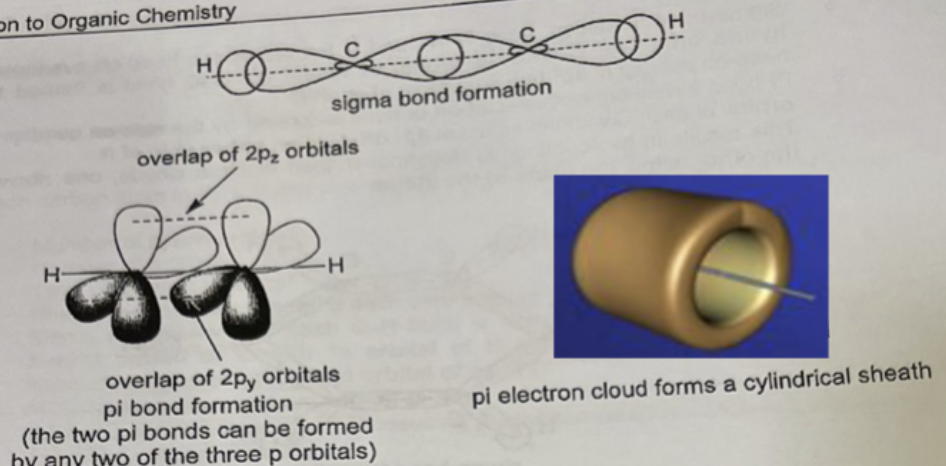
Describe hybridisation in Ethyne, C2H2
Each carbon atom forms two sigma bonds and two pi bonds:
Number of hybrid orbitals: 2
Number of p orbitals: 2
Type of hybridisation: sp
Shape: linear around each carbon atom, bond angle = 180°
Sigma bond formation: each C-H bond is formed by the head-on overlap of sp hybrid orbital of C with 1s orbital of H and the C-C bond is formed by the head-on overlap of sp hybrid orbital of each C
Pi bond formation: one carbon-carbon pi bond is formed by the side-on overlap of 2py orbital of each C laterally and the other carbon-carbon pi bond is formed by the side-on overlap of 2pz orbital of each C laterally
Pi electron cloud forms a cylindrical sheath around the axis joining the carbon atoms
Define substituent
A substituent (usually alkyl or aryl) refers to an atom or group of atoms that takes the place of a hydrogen atom on the hydrocarbon chain
Describe alkyl group
Is a type of substituent derived from alkanes by removing a hydrogen atom
Group of carbon and hydrogen having general formula CnH2n+1
Denoted by letter R
Name: follows corresponding alkane but with -yl
Eg. Methyl , Ethyl
Describe aryl group
Is a type of substituent derived from an aromatic ring (such as a benzene ring) by removing a hydrogen atom
Some organic compounds are classified based on how many substituents are joined to the carbon atom bearing the functional group
Draw hybridisation of ethane
Draw hybridisation ethene
Draw hybridisation of benzene
Draw hybridisation ethyne