3.1.2 - carbohydrates
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
what are monosaccharrides
the molecules from which larger carbs are made
3 examples of common monosaccharrides
glucose
galactose
fructose
what does a condensation reaction between 2 monosaccharides form
glycosidic bond
what is formed by the condensation of two monosaccharides
disaccharides
EXAMPLES OF FORMATIONS OF DISACCHARIDES:
what is formed by the condensation of two α - glucose molecules
maltose
EXAMPLES OF FORMATIONS OF DISACCHARIDES:
what is formed by the condensation of a glucose molecule and a fructose molecule
sucrose
EXAMPLES OF FORMATIONS OF DISACCHARIDES:
what is formed by the condensation of a glucose molecule and a galactose molecule
lactose
what is an isomer
a molecule that has the same chemical formula (in this context, as glucose (C₆H₁₂O₆)) but a different arrangement of atoms
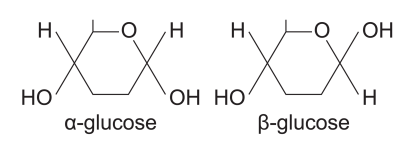
how many isomers does glucose have
what are these called
diagram
2
α-glucose and β-glucose
what are polysaccharrides formed by
the condensation of MANY glucose units
give 4 polysaccharides
starch
glycogen
galactose
amylose
glycogen and starch are formed by the condensation of…
α-glucose
cellulose is formed by the condensation of…
β-glucose
KNOW THE BASIC STRUCTURE AND FUNCTIONS OF CELLULOSE, and the relationship of structure to function of these (in animal and plant cells)
starch is a c________ p___________________ made up of 2 s_________ p_____________________: a___________ and a_______________.
starch is a complex polysaccharride made up of 2 simpler polysaccharrides: amylose and amylopectin.
what monomer is amylose and amylopectin only made up of?
α-glucose monomers
structure of amylose
bonding of amylose
unbranched, coiled helix (helical structure)
monomers joined only by α-1,4 glycosidic bonds
structure of amylopectin
bonding of amylopectin
branched structure
monomers joined by both α-1,4 and α-1,6 glycosidic bonds
how is the structure of starch suited to its function? (reference both amylose and amylopectin)
amylose = helical structure makes it compact, so it can store lots of glucose in a small space
amylopectin = branched chain, so makes it easier for enzymes (e.g. amylase) to access and act on the branched ends simultaneously and break starch down —→ gluc. monomers (energy) can be released very rapidly when needed
how is starch being insoluble suited to its function?
doesn’t dissolve / create osmotic pressure in cells —→ could damage them
keeps starch as a stable, non-reactive energy source
what would happen if starch were soluble?
would dissolve in the cytoplasm, increasing solute conc.
would cause water to enter the cell by osmosis
Too much water = pressure builds up → cell could swell and burst (esp. in animal cells without a strong cell wall)
how is starch being a large molecule suited to its function?
ideal for long-term storage
lots of gluc. can be stored in one molecule
prevents it from diffusing out the cell
what organisms is glycogen found in
in animals, glycogen is stored as s______ g__________ mainly in the m_______ and l______
in animals and bacteria (but never plant cells)
in animals, glycogen is stored as small granules mainly in the muscles and liver
glycogen has a similar structure to starch, but how does it differ?
has shorter chains
is more highly branched
how does the structure of glycogen suit its function?
compact —→ a lot can be stored in a small place
insoluble —→ doesn’t draw water into the cells by osmosis + doesn’t diffuse out of cells
more highly branched than starch —→ more ends that can be acted on simultaneously by enzymes —→ is more rapidly broken down into gluc. monomers
in terms of monomers, how does cellulose differ from starch and glycogen
made of monomers of β-glucose (not α-glucose)
in terms of structure, how does cellulose differ from starch
cellulose = has straight, unbranched chains
(unlike starch, which has a coiled, helical structure)
describe the cellulose structure in more detail
straight, unbranched chains run parallel to one another
which allows H bonds to form cross linkages between adjacent chains
what do the extra H bonds do for strengthening the molecule
while each individual H bond adds v. little to the strength of the molecule…
sheer volume of them makes a considerable contribution to strengthening cellulose
where is the -OH on C1 in the β-glucose molecule
where is the -H on C1 in the β-glucose molecule
(it’s opposite for α-glucose molecules)
up, above the ring
down, below the ring
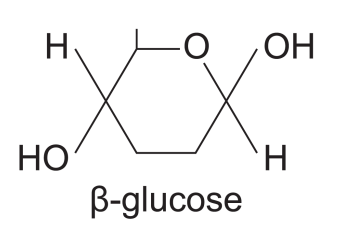
because of this structure, forming glycosidic bonds is difficult.
how do we overcome this?
(add diagram)
alternate gluc. need to be flipped 180 degrees to ensure the OH groups are next to each other.
what are reducing sugars
carbohydrates that can donate electrons (or H atoms) to another chemical, typically Benedict’s reagent (which is used to test for the presence of reducing sugars)
test for reducing sugars? (2 steps)
result?
(1) add benedict’s reagent to sample in a test tube
(2) heat the mixture in water bath at ~80℃ for 2-5 mins
if reducing sugars = present, a brick-red precipitate should form
which monosaccharides are reducing sugars?
give examples
all of them!
glucose, galactose and fructose
which disaccharides are reducing sugars?
maltose
lactose
what does this mean sucrose is?
a non-reducing sugar
define a non-reducing sugar
does not donate electrons to a chemical, and therefore does not give a positive result when reacted with Benedict’s reagent
how would you test for a non-reducing sugar (i.e. if the reducing sugar test was negative?) (4 steps)
(before all this would be the 2 steps for the Benedict’s reagent test, but obvi it would have failed bc no reducing sugar is present !!)
hydrolyse the non-reducing sugar (NRS) by adding dilute HCl to the new sample
heat in a water bath for 5 min to break the NRS into reducing sugars (monosaccharides)
neutralise the acid by slowly adding a weak alkali, like sodium hydrogen carbonate (NaHCO3), until it is neutral. test pH to make sure solution is neutral 7 (Benedict's test requires alkaline conditions)
repeat Benedict’s test (add Ben’s solution again and heat for 2-5 mins)
give the positive and negative result, and what they indicate
POSTIVE RESULT → a brick-red precipitate forms → NRS was present
NEGATIVE RESULT —→ sample remains blue —→ no sugar was present at all (NRS or reducing)
what is the biochemical test for reducing sugars and non-reducing sugars
Benedict’s solution
what is used for the biochemical test for starch
iodine or potassium iodide