graphite c2
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
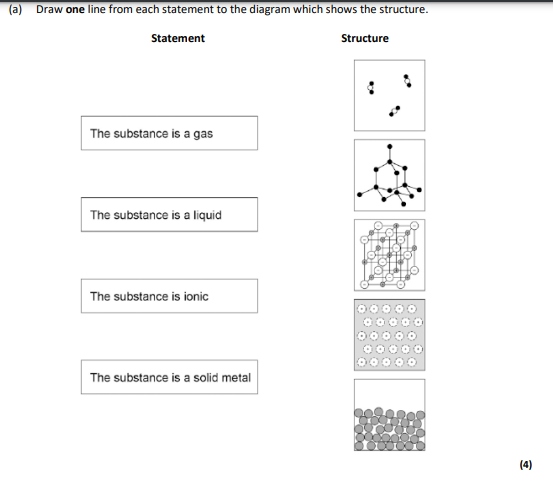
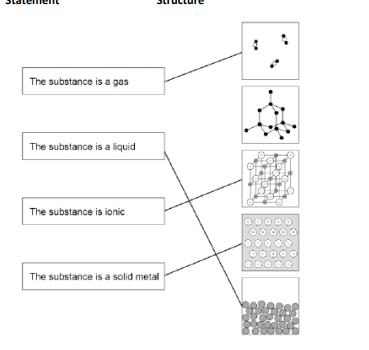
gas-1 liquid-last ionic-3rd solid metal-4th
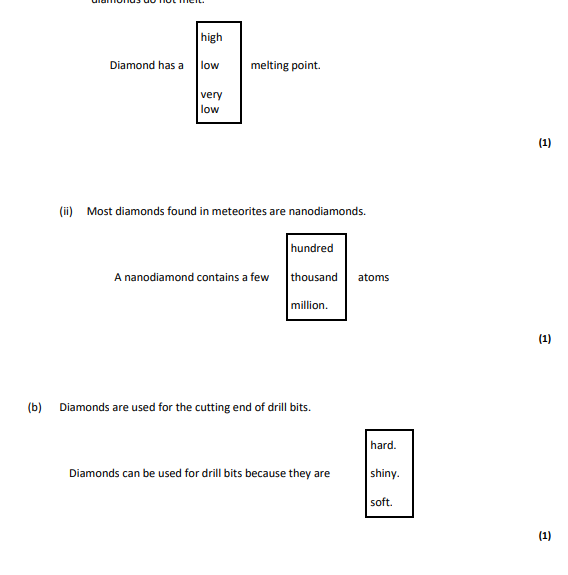
high,100,hard

carbon,It has delocalised electrons
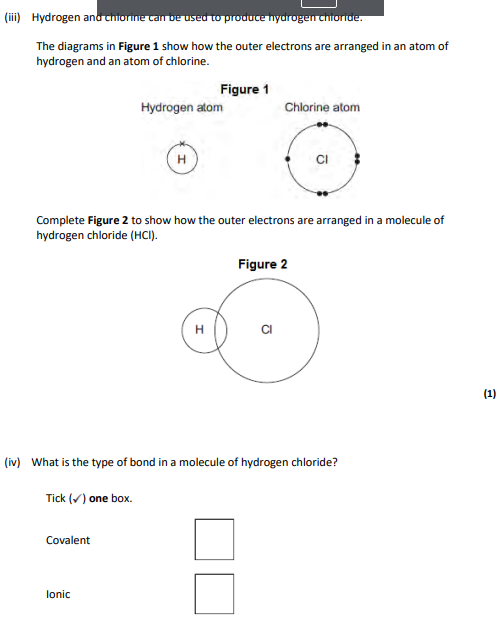
1 bonding pair of electrons 6 unbonded electrons on Cl,covalent
slide,c

strong covalent bonds
high,100,hard
carbon,4
covalent,all
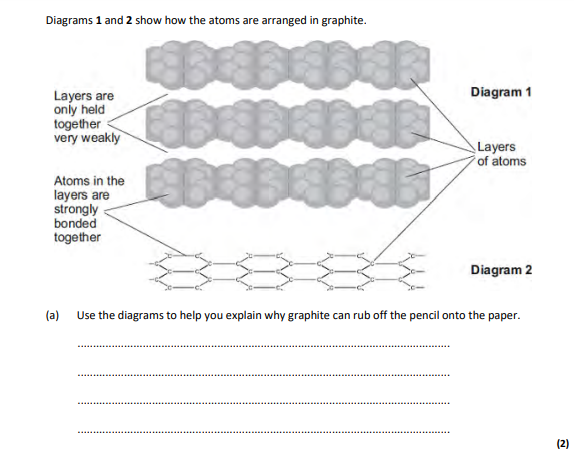
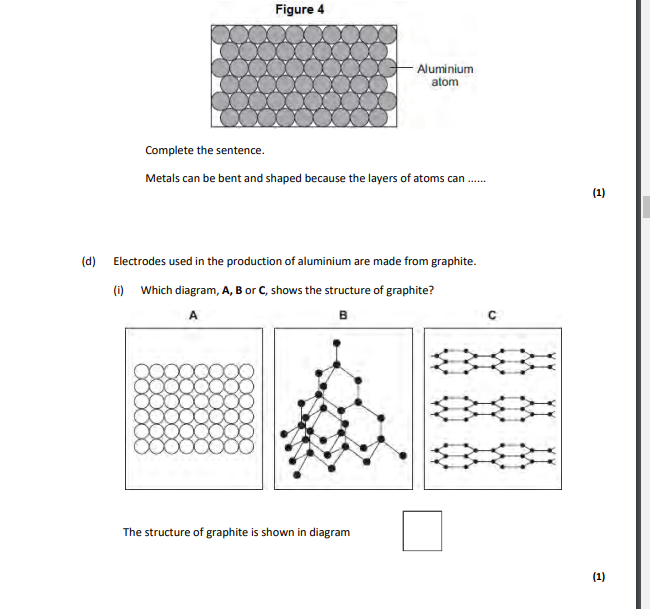
layers,which can slide over each other
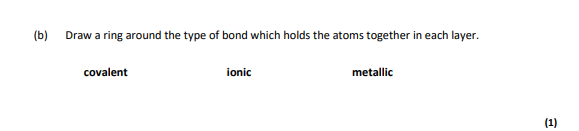
covalent
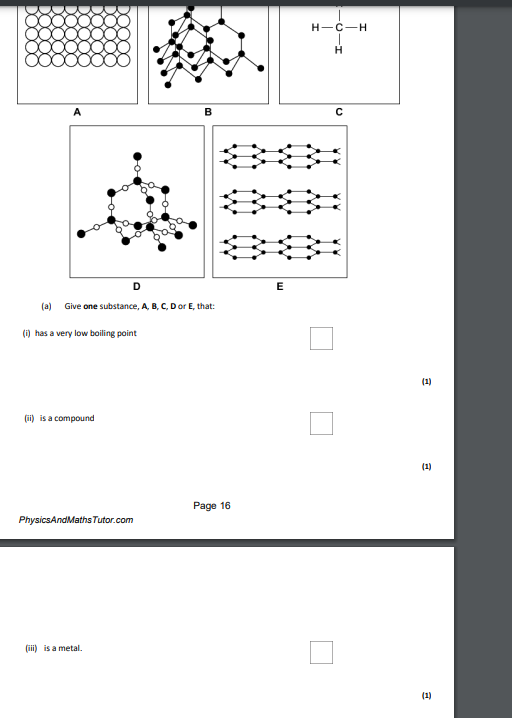
c,d,a

covalent,layers can slide over eachother
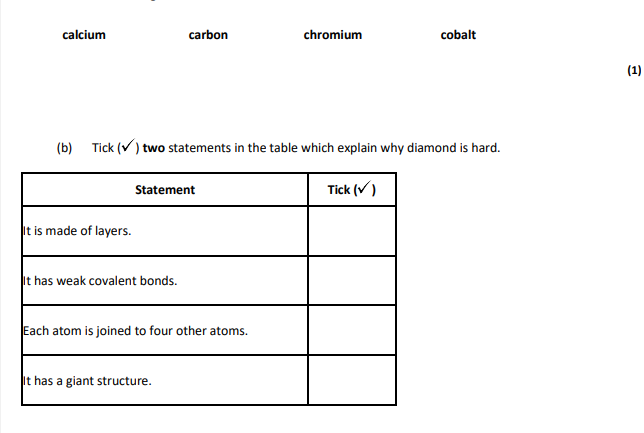
carbon,each atom is joined to four other atoms It has a giant structure
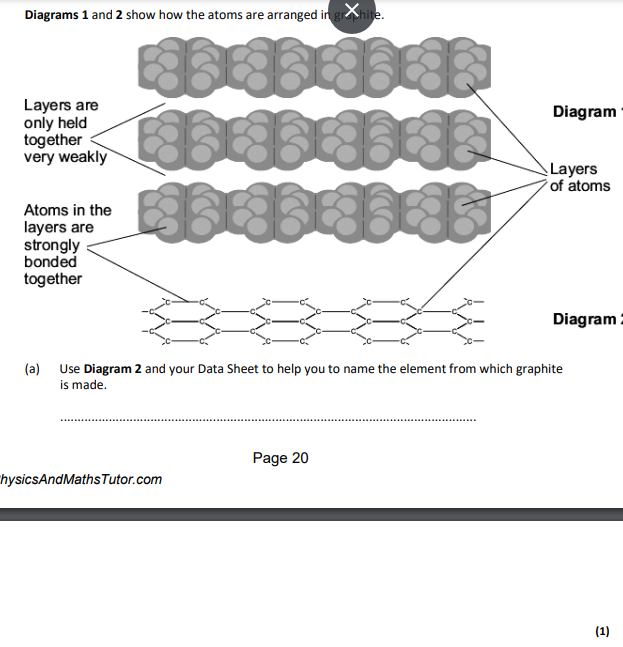
carbon

layers,can slide over each other,covalent
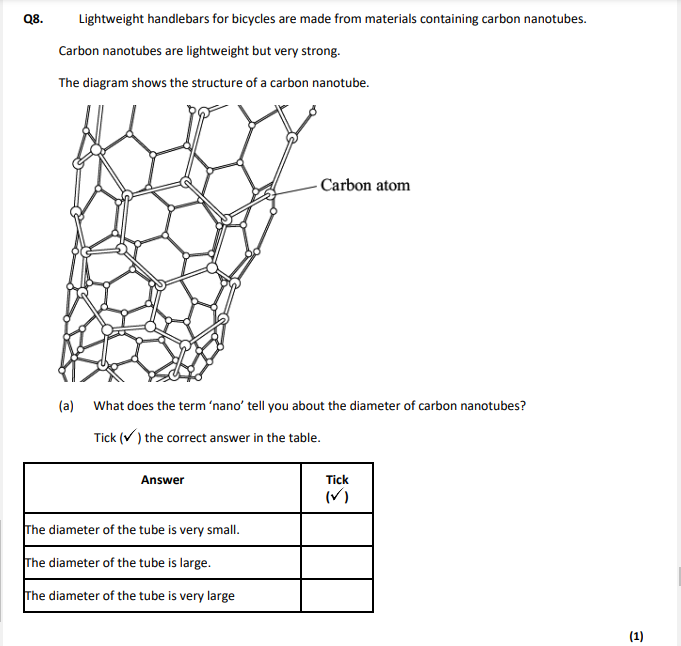
M8. (a) the diameter of the tube is very small
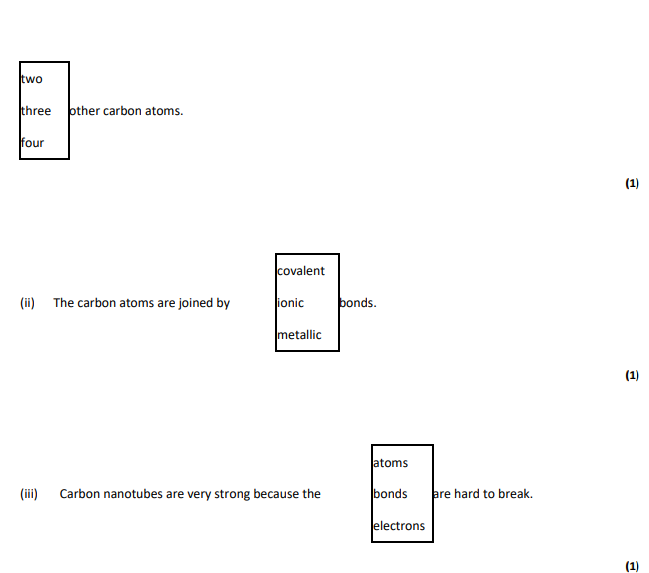
Carbon nanotubes are similar to graphite because each carbon atom is joined to..
3,covalent,bonds
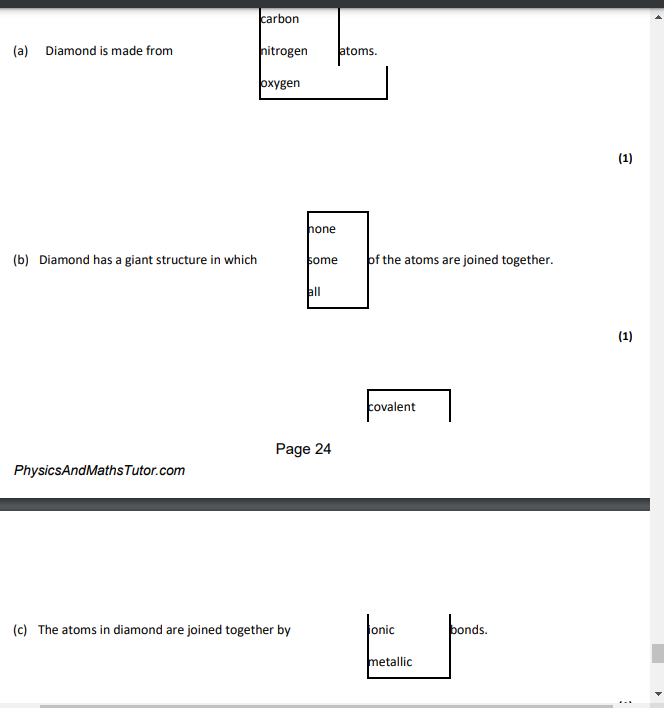
carbon,all,covalent
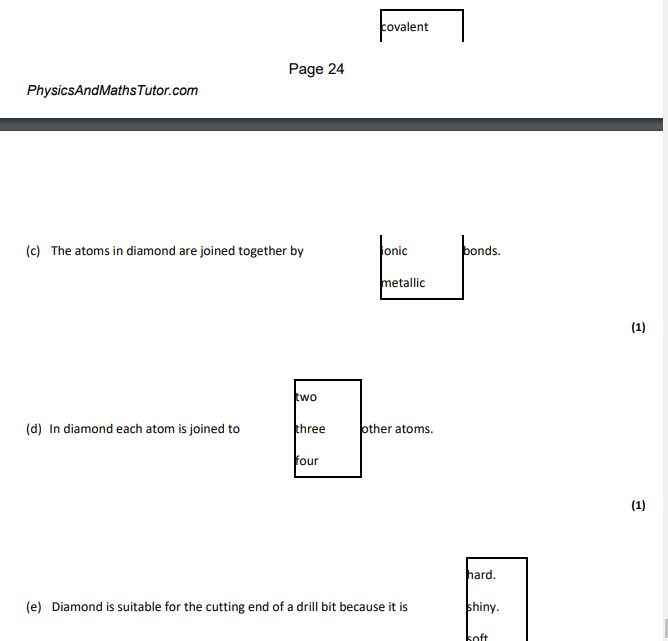
covalent,4,hard
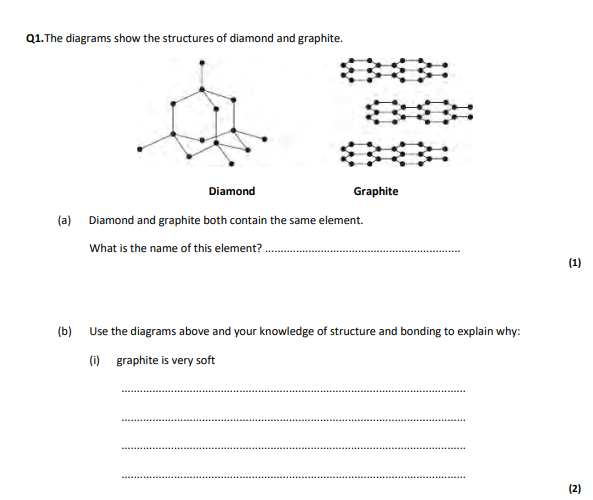
carbon,atoms are in layers that can slide over each other,because between the layers there are only weak forces

because each atom forms four covalent bonds and covalent bonds are strong. q2Because graphite has delocalised electrons,which can move through the whole structure
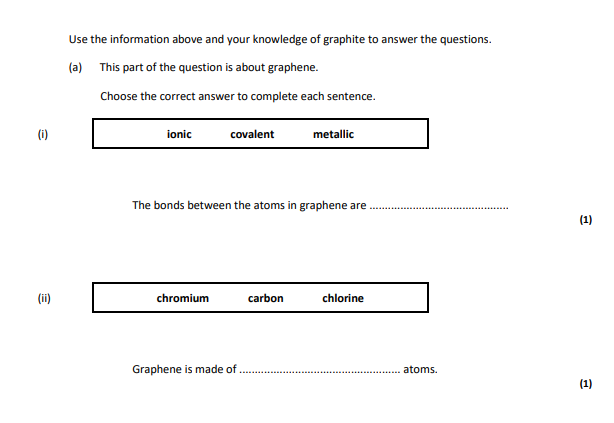
covalent,carbon
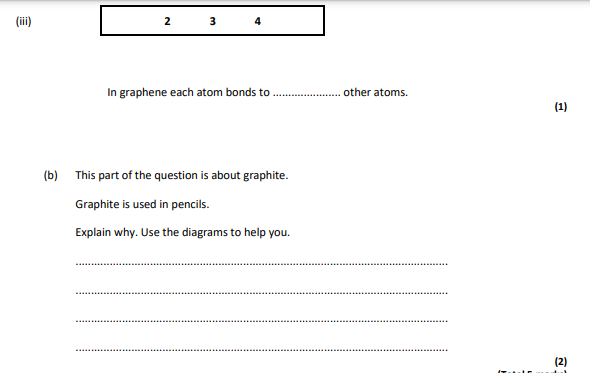
3,layers can slide,graphite left on the paper
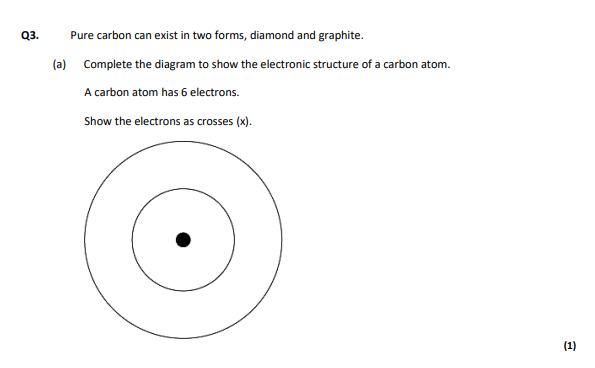
2,4 on shels
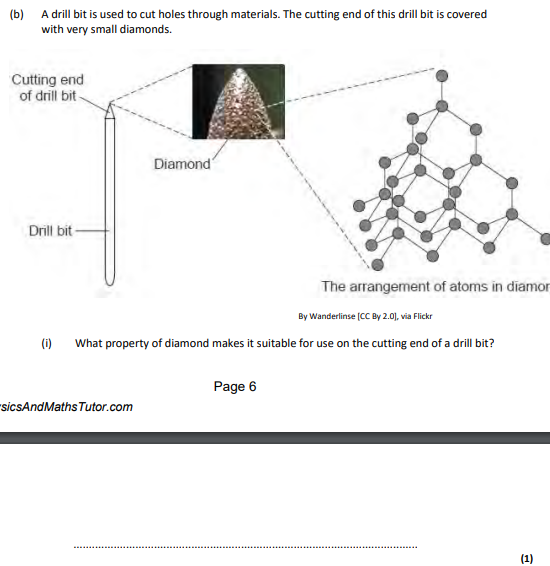
hard

lattice,covalent bonds,each carbon forms four bonds

graphite has delocalised electrons which can carry charge but diamond has no delocalised elecrons

(i) explain why graphene is strong;
giant lattice,atoms in graphene are covalently bonded,and covalent bonds are strong

there are free electrons,because one free electron per atom

because there are weak forces between molecules so layers can slide

Use your knowledge of the bonding in graphite and the photograph of the structure to help you to explain, as fully as you can: (a) (i) why graphene is strong;
it has a giant structure,t has covalent bonds ,the bonds are strong

(a) Graphite is softer than diamond.
because the layers (of carbon atoms) in graphite can slide,because there are only weak intermolecular forces,however, in diamond, each carbon atom is strongly bonded to 4 others so no carbon able to slide
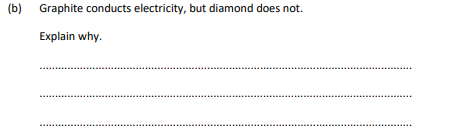
Graphite conducts electricity, but diamond does not.
because graphite has delocalised electrons,which can carry charge,but, diamond has no delocalised electrons

Suggest why a sheet of graphite which has a large number of carbon layers would not be suitable for the touchscreen.
opaque
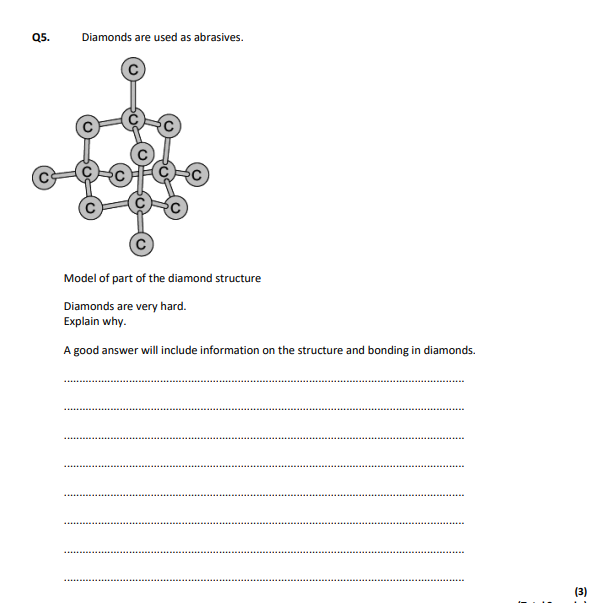
giant structure,covalent bonds,very strong bonds,each carbon joined to four others
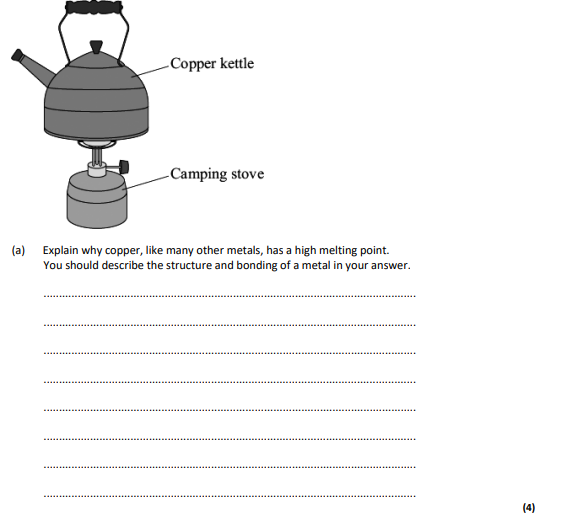
explain why copper, like many other metals, has a high melting point. You should describe the structure and bonding of a metal in your answer.
giant structure,positive ions,delocalised electrons,bonds between atoms are strong

What does the term ‘nano’ tell you about the carbon nanotubes? . (1) (ii) Like graphite, each carbon atom is joined to three other carbon atoms. Explain why the carbon nanotube can conduct electricity.
they are very small,delocalised electrons,electron carry charge

in layers,layers can slide over each other,giant structure,covalent (bonds),strong bonds
