PH8. Radioactivity
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
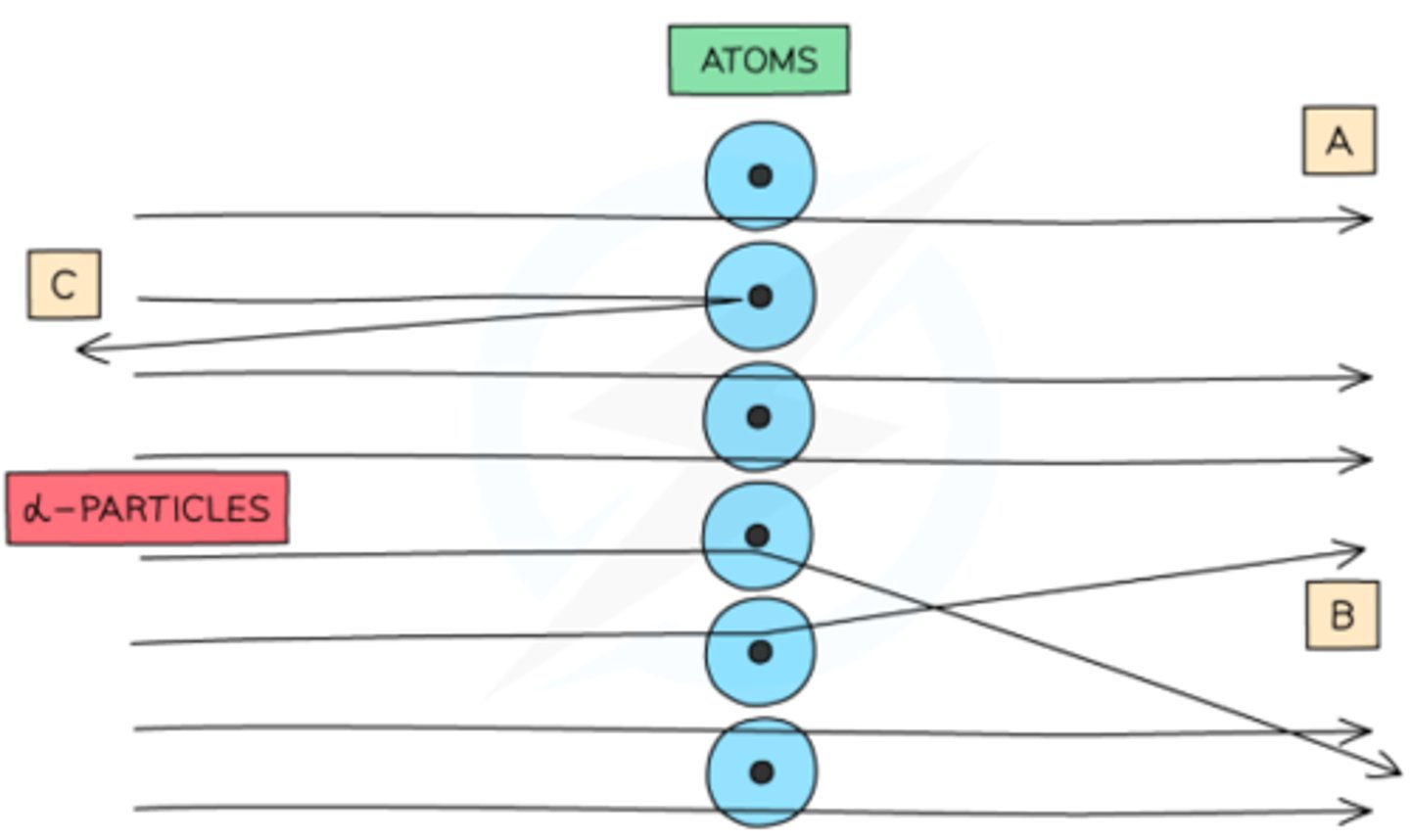
How was the nucleus discovered by Rutherford?
Rutherford fired a narrow beam of fast-moving, positively charged α particles at a thin gold foil and discovered that:
- Most α particles passed straight through
→ Atom is mostly empty space
- Some α particles were deflected at angles <90°
→ Nucleus is small, dense, and positively charged so repels α particles due to electrostatic forces
- A few α particles bounced back at angles >90°
→ Nucleus contains most of the atom’s mass
How was Rutherford's model different to the Plum Pudding model, and why was the nucleus not discovered earlier?
- Old model had a large positively charged sphere with negative charges inside
- New model had a small positively charged dense nucleus
- A concentrated nucleus wasn't discovered earlier due to lack of advanced techniques like high-energy α particles and precise measurements for particle scattering
How did Rutherford estimate the size of the nucleus?
- Probability of large-angle deflection (>90∘) is about 1 in 10000n, where n is the number of atomic layers in the foil, typically 10000
- Area ratio of nucleus to atom = πd²/4 : πD²/4
- d² = D²/10000n → d = D/10000
What are the properties of alpha radiation?
Nature: Helium nuclei (2 protons+2 neutrons)
Range in Air: Fixed range, up to 100mm
Deflection in Magnetic Field: Deflected in the opposite direction to β particles but deflect less due to higher mass
Absorption: Stopped by paper or thin metal foil
Ionisation: Strongly ionising
What are the properties of beta radiation?
Nature: High-speed electrons (β−) or positrons (β+)
Range in Air: Up to 1m. Depends on initial kinetic energy
Deflection in Magnetic Field: Deflected in the opposite direction to α particles but more easily deflected due to lower mass
Absorption: Stopped by 4mm of aluminium or 2–3mm of steel
Ionisation: Weakly ionising
What are the properties of gamma radiation?
Nature: High-energy photons
Range in Air: Unlimited range but intensity decreases with distance following the inverse square law
Deflection in Magnetic Field: Not deflected due to no charge
Absorption: Reduced significantly by several centimetres of lead or 5cm of steel
Ionisation: Very weak ionising effect
Why is ionising radiation dangerous?
- Damages living cells by knocking electrons off atoms, creating ions
- Damages DNA directly or indirectly via free radicals, leading to mutations, cancerous growth, or cell malfunction
- Destroys cell membranes, causing cell death or tissue damage
- No safe threshold as even low doses can cause damage
What is the inverse square law for gamma radiation?
- The intensity I of gamma radiation decreases with the square of the distance r from the source, as radiation spreads uniformly over the surface area of a sphere (4πr²)
- So I = radiation energy per sec/total area = nhf/4πr²
- Therefore the inverse square law states I=k/r², where k= nhf/4π
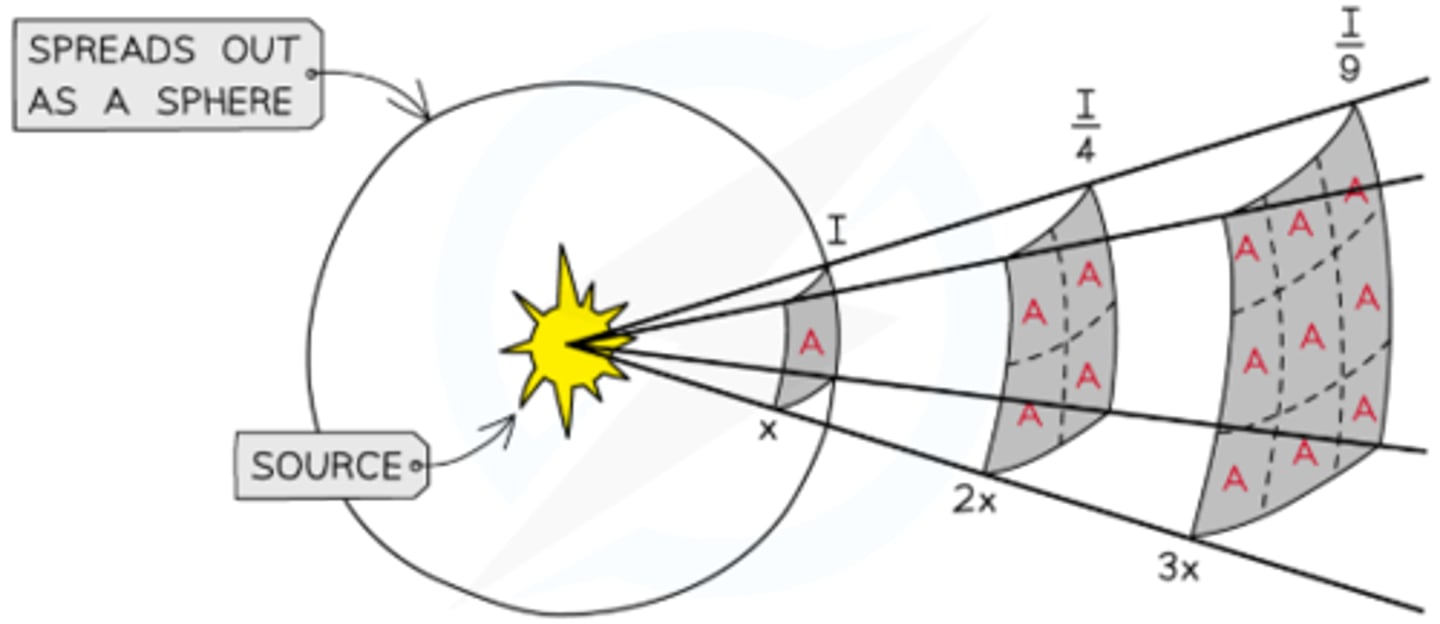
How is the inverse square law for gamma radiation verified?
- Place a gamma source at the center of a spherical setup
- Use a Geiger-Müller tube to measure count rate and subtract background radiation
- Repeat for different distances using a ruler
- Plot count rate against 1/distance²
- Straight line through the origin confirms the law
- Use a large range of distances for accuracy
- For accuracy, measure a long time with a large range of distances, and repeat readings to find a mean
What happens during electron capture in a nucleus?
- A proton-rich nucleus captures one of its inner-shell electrons, converting a proton into a neutron and emitting a gamma ray and electron neutrino (νe)
- The inner-shell vacancy is filled by an outer-shell electron, emitting an X-ray photon
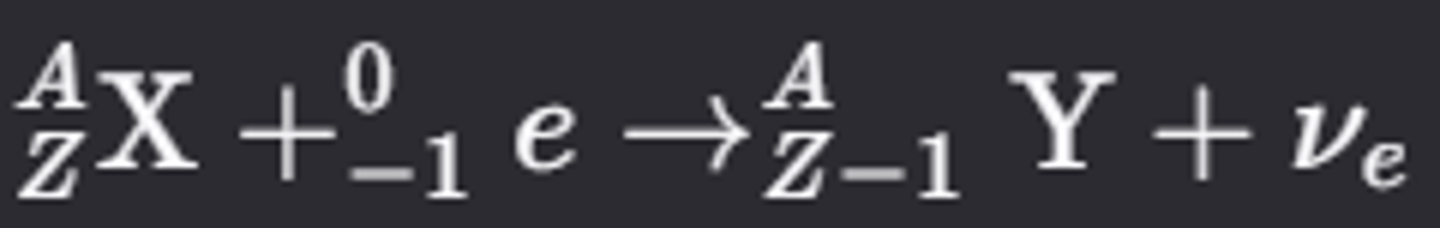
What is the decay process of a free neutron?
- A free neutron decays into a proton by emitting a beta-minus particle (0 −1β) and an antineutrino (νˉe)
- The proton is stable and does not decay further
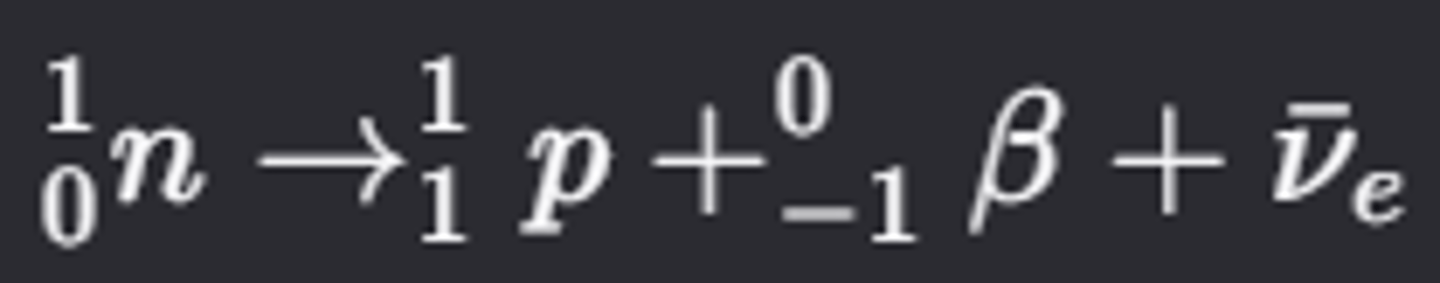
What are the somatic and genetic effects of ionising radiation?
- Somatic effects impact the exposed individual by damaging DNA, causing cell death and cancerous tumour formation
- Genetic effects occur when mutations in sex cells (egg or sperm) are passed to future generations, leading to abnormalities in offspring
What factors determine whether α, β, or γ radiation is most dangerous?
α Radiation: Highly ionising but cannot penetrate skin externally so most dangerous internally like if ingested or inhaled
β Radiation: Moderately penetrating and ionising but harmful if close to the body or inside it
γ Radiation: Weakly ionising but highly penetrating, posing less risk internally but more danger externally due to its ability to pass through the body
How can exposure to ionising radiation be reduced?
- Use tongs to maintain distance
- Store radioactive materials in sealed lead containers to prevent inhalation and block gamma
- Wear film badges to monitor exposure and reduce dose
- These precautions reduce risk of contamination and irradiation to the body
What is background radiation, and how does it vary?
- Background radiation is naturally occurring ionising radiation from cosmic rays or radioactive materials in rocks, soil, air, and radon gas
- Varies in levels depending on location, like accumulation of radon gas in poorly ventilated areas
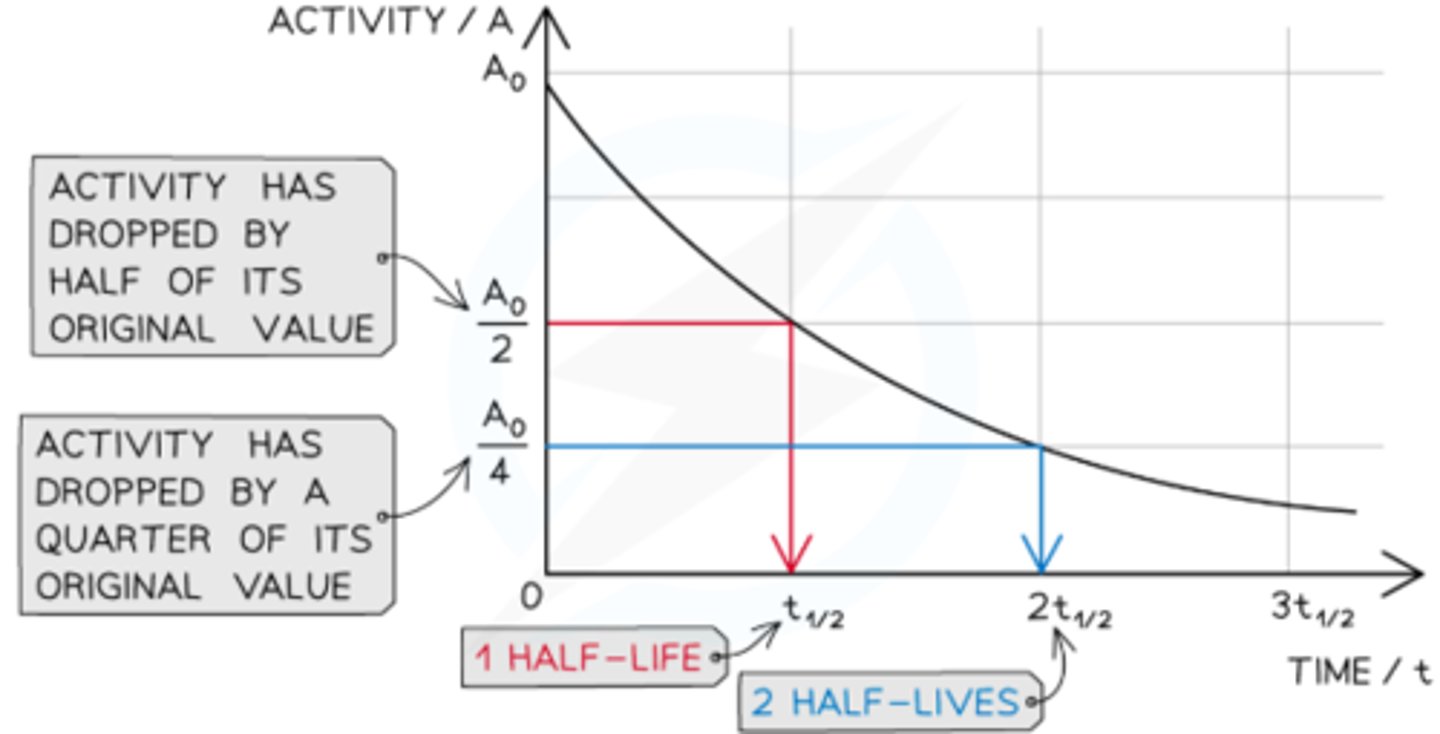
What is half-life?
The time taken for the mass/activity/number of nuclei of a radioactive isotope to halve
How is carbon dating performed?
- Measures the decay of carbon-14 (14C) in dead organic material
- Living organisms absorb 14C which after death decays with a half-life of 5570 years
- The ratio of 14C in dead wood to living wood gives 0.5^n (number of half-lives)
- If the ratio is 0.25, n = log0.5^0.25 = 2, so age = 2×5570 = 11,140 years
How is argon dating performed?
- Measures the decay of potassium-40 (40K) into argon-40 (40Ar) with a half-life of 1250 million years
- Potassium-40 (40K) also decays into calcium-40 (40Ca) via beta emission, which is eight times more probable than electron capture
- If there is one argon-40 atom for every N potassium-40 atoms now present, the original number of potassium atoms was N+9
- Use N/N0 = 0.5^n to calculate age = number of half-lives×half-life
What are radioactive tracers?
- A radioactive tracer is a substance containing a radioactive isotope used to track its movement through a system
- Half-life is stable enough for measurements but short enough to decay quickly
- Emits beta or gamma radiation for external detection
What factors determine the choice of a radioactive isotope for a specific application?
- Half-life should be long enough for stability during use but short enough to decay quickly after use
- Type of radiation required; Beta or gamma for external detection, alpha for internal
- Decay Product produced should be stable product to avoid further complications
- Biochemical Suitability is important for medical applications as the isotope must not harm living organisms
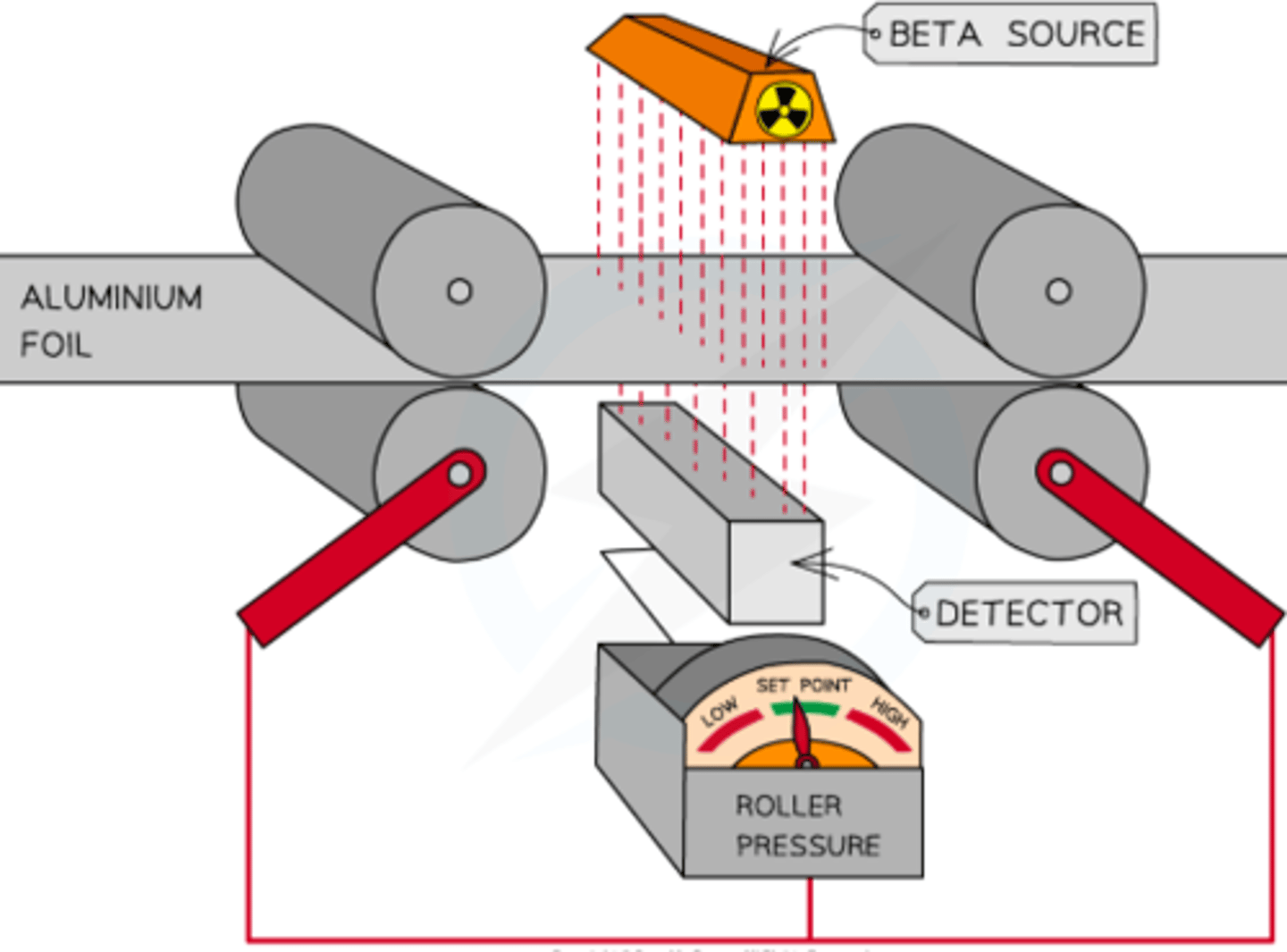
How does thickness monitoring using beta radiation work in industrial processes?
- A beta source is placed above the metal sheet, and a detector below measures radiation
- If the foil is too thick, less radiation reaches the detector, triggering a feedback system to adjust the rollers to make the foil thinner, allowing consistent thickness
- Gamma radiation passes through completely, and alpha radiation is fully absorbed, making beta radiation ideal
What can the neutron-to-proton ratio tell you about radioactive isotopes?
Indicates whether a nucleus is stable or unstable:
- Too many neutrons → β⁻ emission
- Too many protons → β⁺ emission
- Too many nucleons → α emission to reduce both protons and neutrons
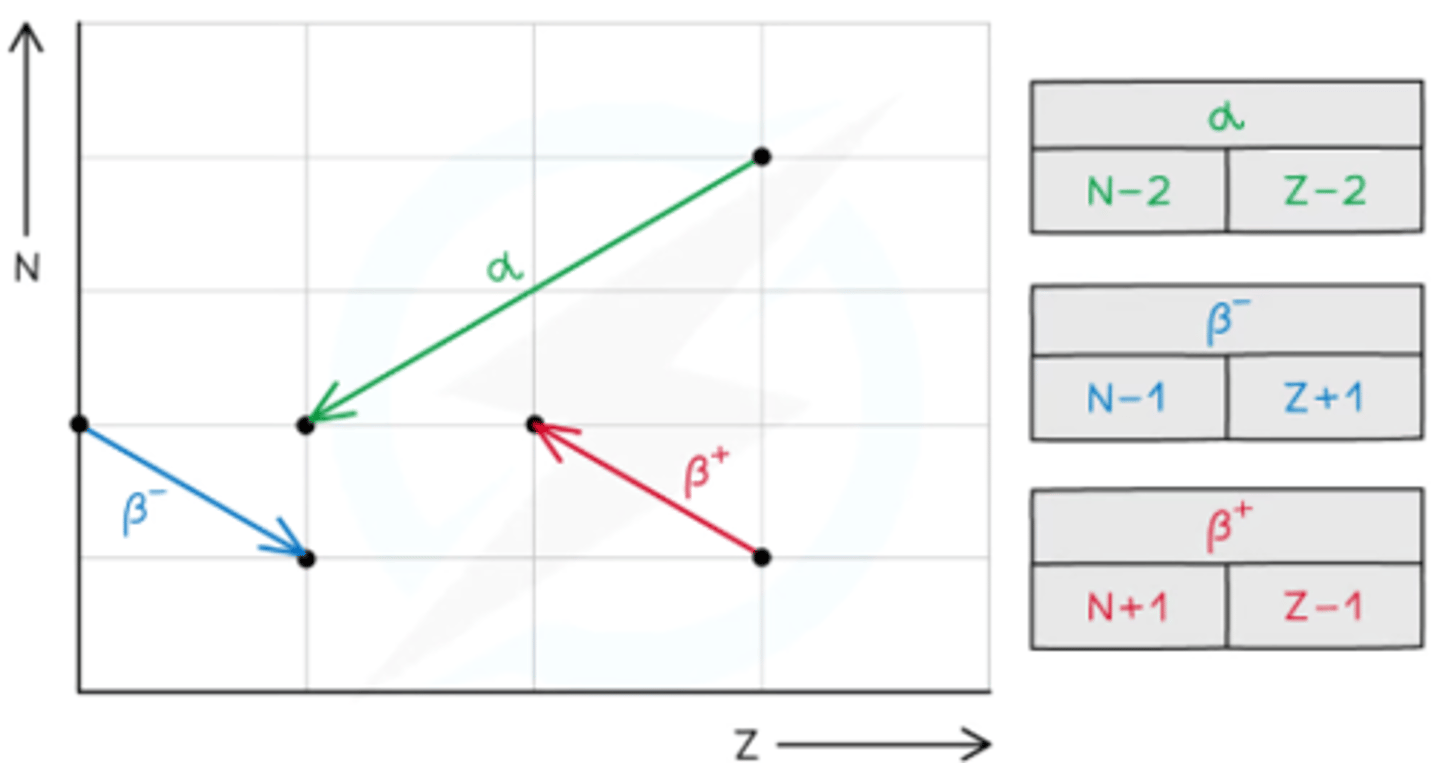
Why don't naturally occurring isotopes emit β⁺ radiation?
- Naturally occurring isotopes have a high neutron-to-proton ratio, leading to β⁻ emission for stability
- β⁺ emission would make them even more neutron-rich and unstable, so it is not observed
What happens to an unstable nucleus that emits alpha or beta radiation?
After α or β emission, the nucleus transitions from its excited state to its ground state by emitting γ photons at specific energy levels, allowing it to lose excess energy
What is a radioactive series?
A series of isotopic changes where an unstable nucleus decays via α, β⁻, or β⁺ emission until it becomes stable, common in naturally occurring isotopes
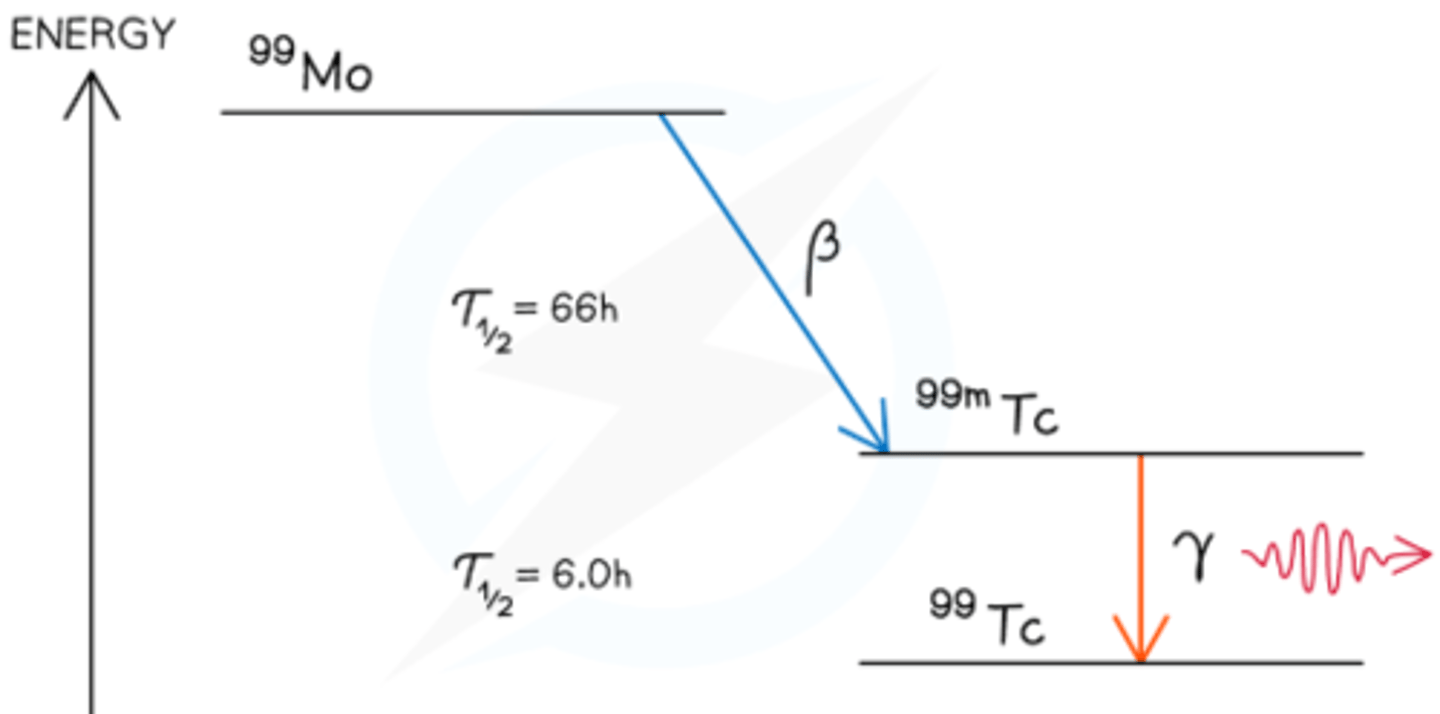
How does the technetium generator work, and what is its application?
- A technetium generator contains molybdenum-99, which decays into metastable technetium-99m via β⁻ emission
- 99mTc has a half-life of 6 hours and decays to its ground state by emitting γ radiation
- Used in medical diagnostics to monitor blood flow or image internal organs/bones, like locating bone deposits using 99mTc phosphate tracers
Why must tracer samples be used as soon as they are produced?
- Activity must be large enough to be differentiated from the background radiation
- So that it isn't absorbed by the body