Pulmonology Combined- Week 2
1/384
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
385 Terms
gas exchange
-O2 and CO2 transfer at the level of an alveolus
-operation of the lungs as a whole
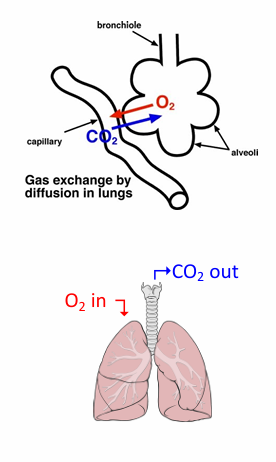
if gas exchange was perfect
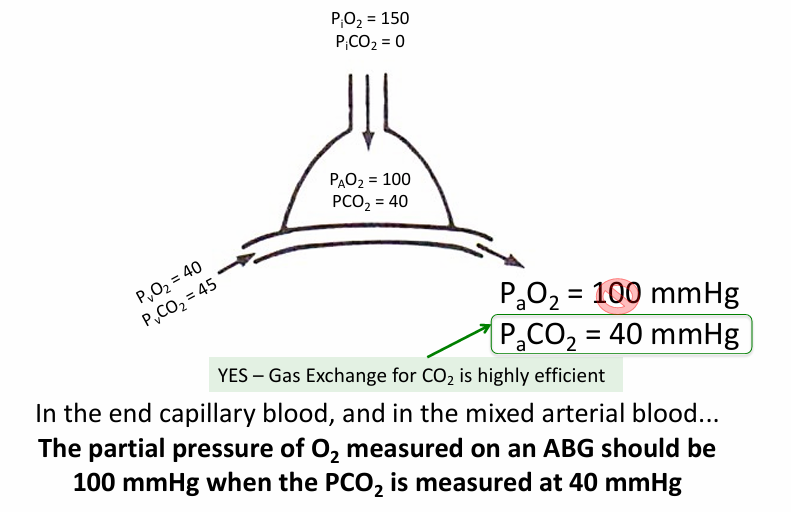
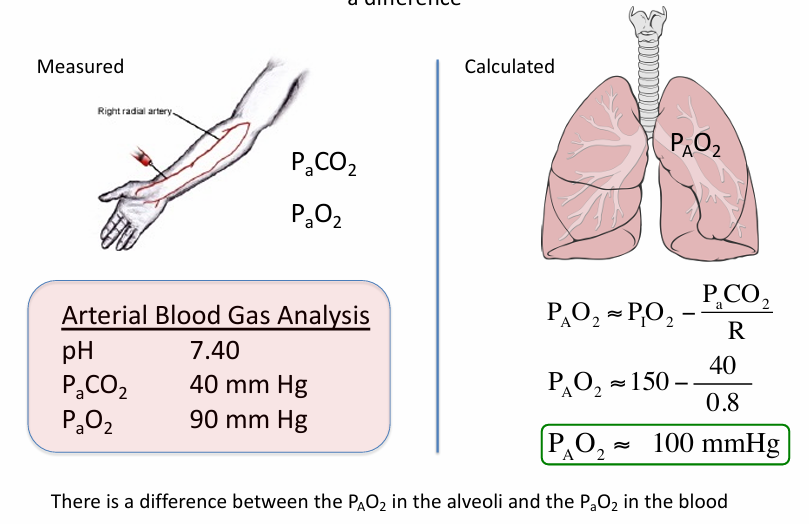
PAO2 in alveoli v PaO2 in blood
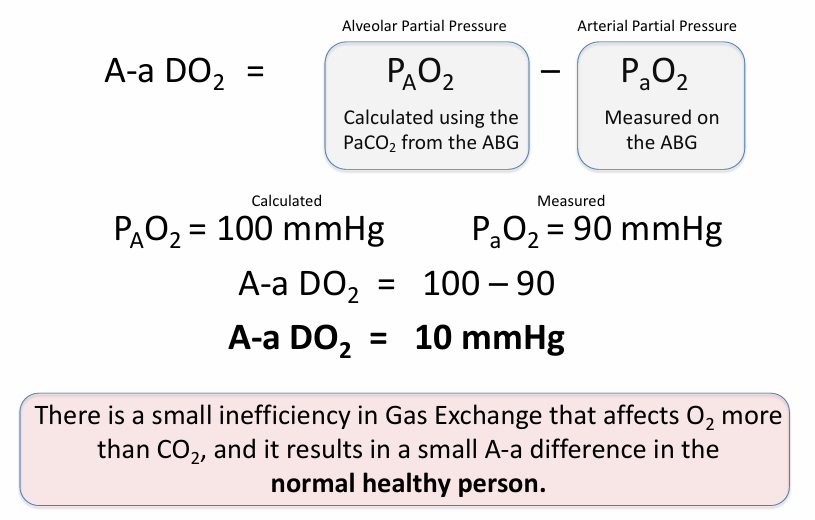
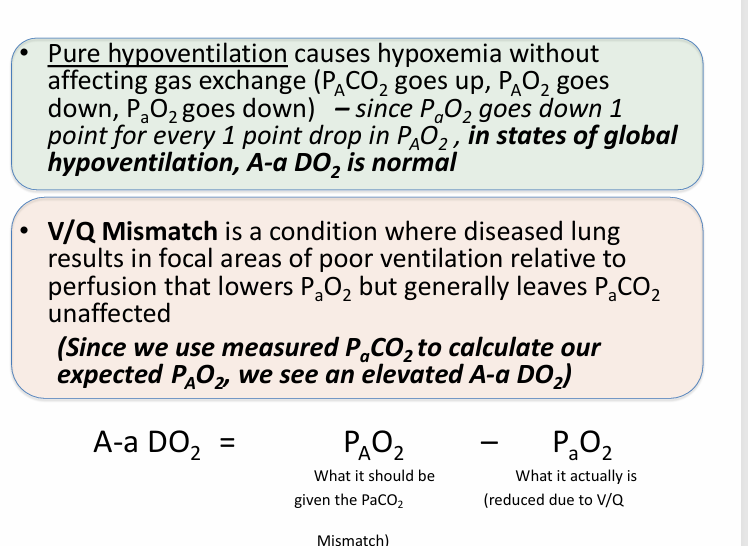
A-a gradient (A-a DO2)
-normal A-a DO2 increases with age
-age/4+4 is a general approximation
ratio of ventilation to perfusion
-key to gas exchange

V/Q relationships
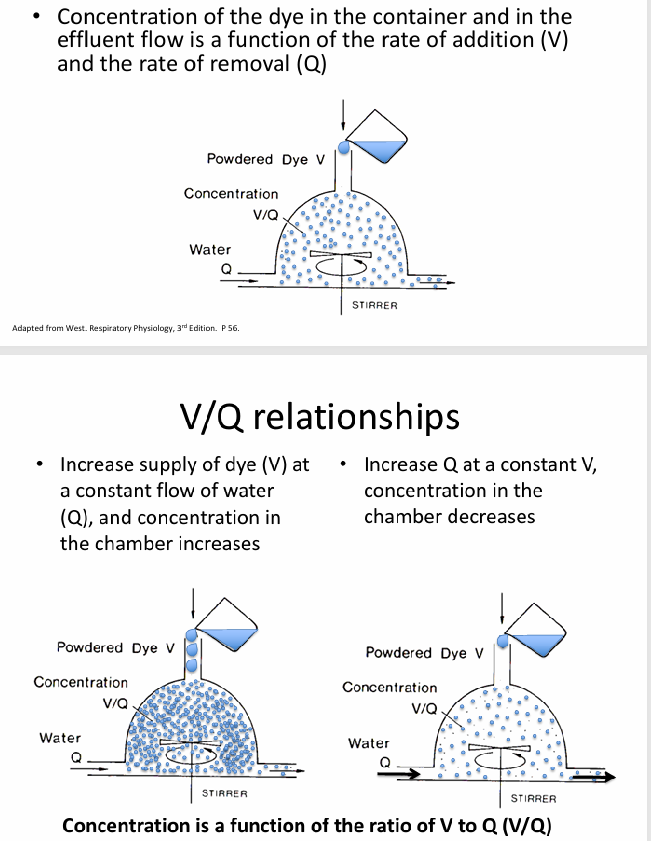
V/Q relationships- ventilation, capillary blood flow
-ventilation of atmospheric O2 is source of O2 in alveolus (V)
-capillary blood flow carries O2 away (Q)
-concentration of O2 in the alveolus (PAO2) is a function of V/Q ratio
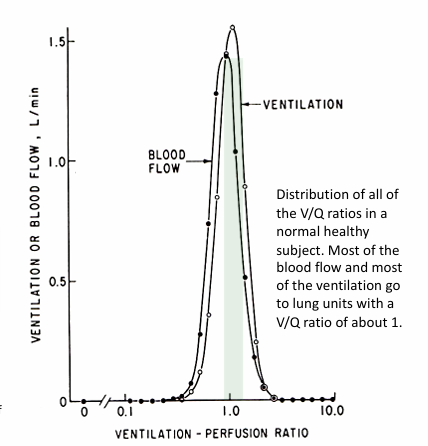
V/Q in healthy lungs
-most alveoli have a V/Q relationship that is at or near 1
-ventilation and perfusion are relatively evenly matched in the majority of alveoli
-some alveoli have low V/Q (<1)
-some alveoli have high V/Q (>1)
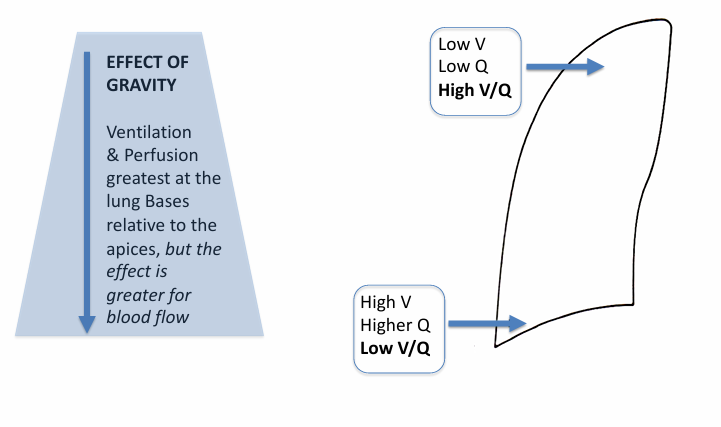
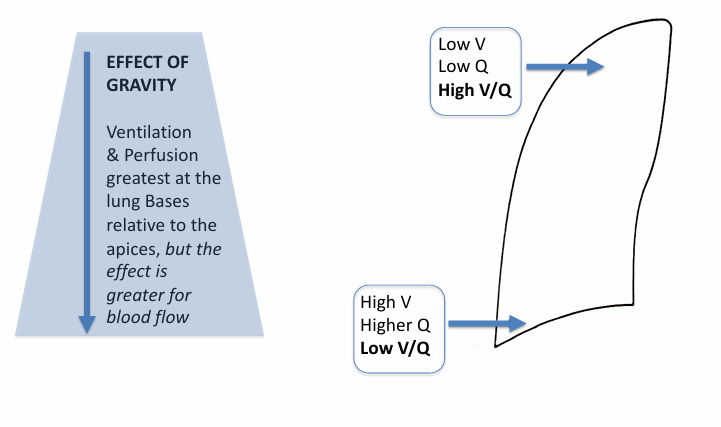
normal V/Q differences in the upright lung
regional gas exchange in the lung
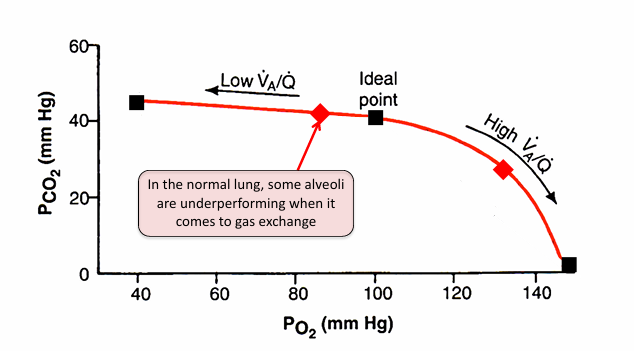
range of V/Q lung units in normal lung
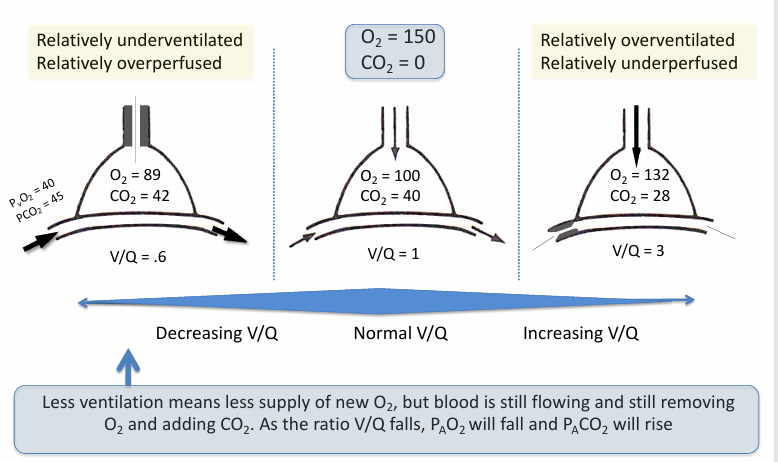
alveolar O2 and PCO2 relationship in normal lungs
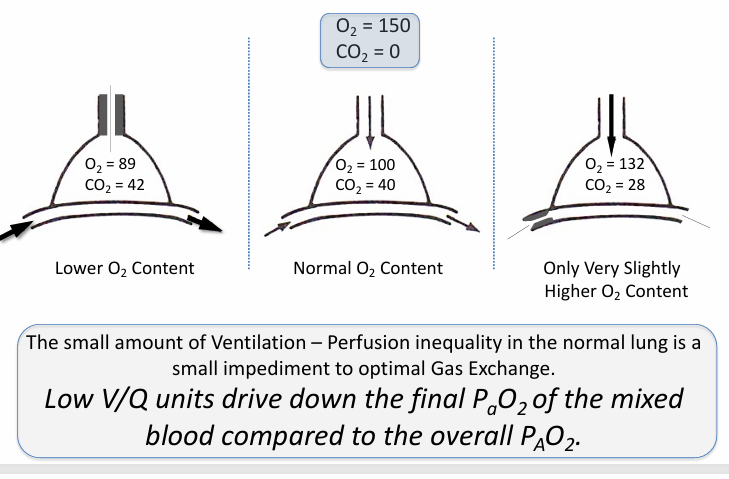
range of V/Q lung units in normal lung- lower v higher O2 content
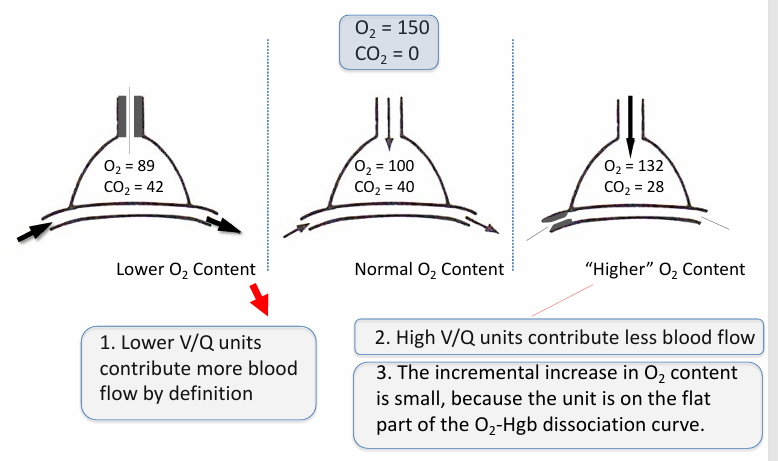
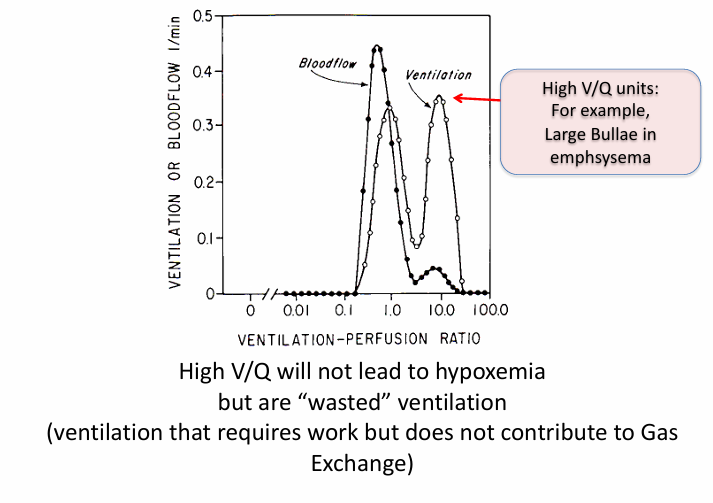
higher V/Q units
-can’t boost O2 content by very much
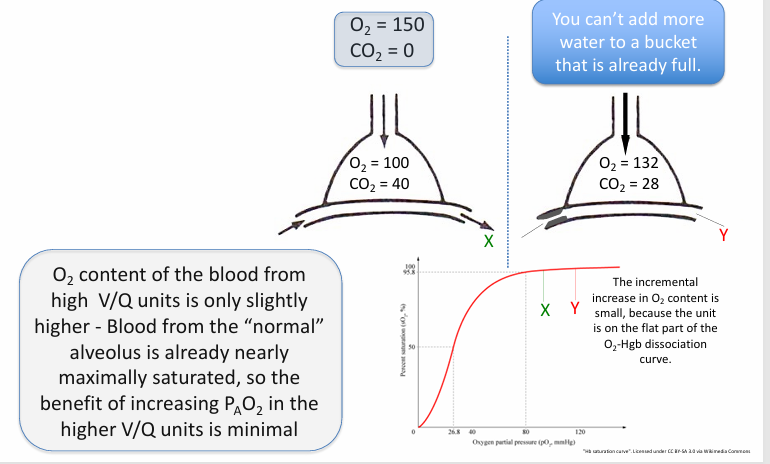
in normal, healthy lungs, heterogeneity in V/Q matching…
-drives down the arterial PaO2
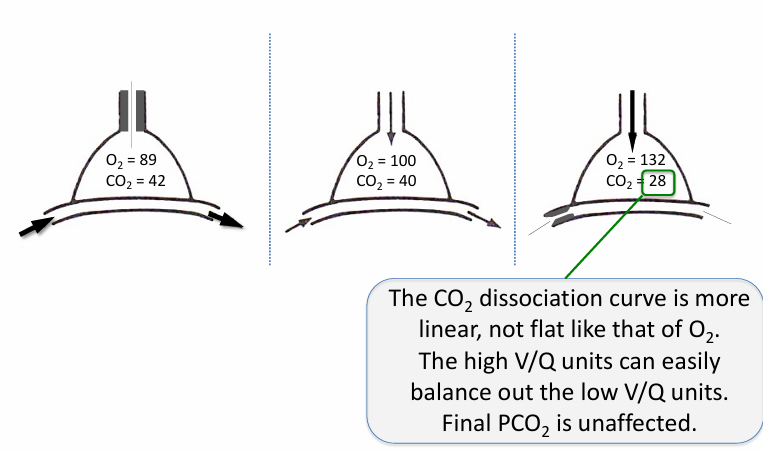
V/Q heterogeneity and PCO2 in the normal lung
-matters less
other reasons why A-aDO2 ~10 in a normal healthy adult (breathing room air)
-diffusion of O2 into the capillary falls short of 100% equilibration at the end of the capillary-alveolar interface
-there is a small amount of physiologic shunt: bronchial arteries draining into pulmonary veins, thebesian veins drain into the LV (deoxygenated blood mixed back into the oxygenated blood pool)
terms- V/Q matching, A-a gradient, V/Q mismatch
-V/Q matching: lungs achieve efficient gas exchange by tightly matching ventilation (V) and perfusion (Q)
-A-a gradient: normal lungs have a small A-a difference
-V/Q mismatch: many diseases of the lungs negatively affect ventilation or perfusion (or both) and therefore affect the lungs ability to match V and Q, thereby negatively impacting gas exchange
-diseases that cause V/Q mismatch will result in hypoxemia, and this will be reflected in a measured increase in the A-a gradient
-diseases that negatively effect gas exchange in other ways can cause an increase in the A-a gradient (multiple causes)
A-a gradient in disease
A-a gradient in disease- focal effect
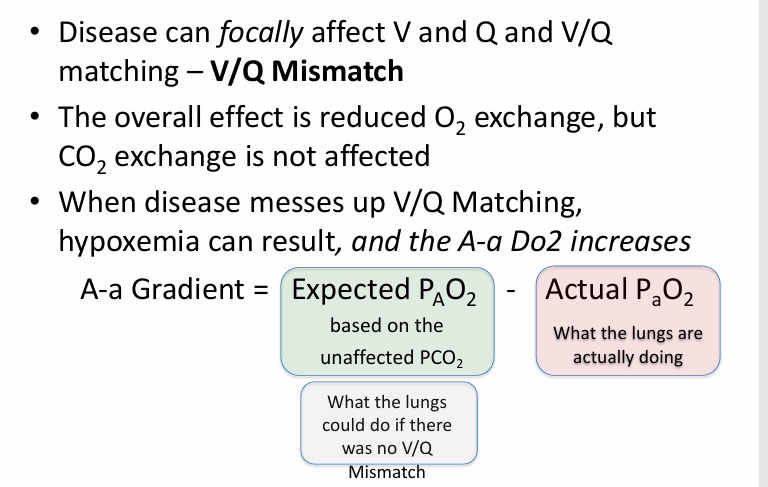
V/Q mismatch causes
-an A-a gradient
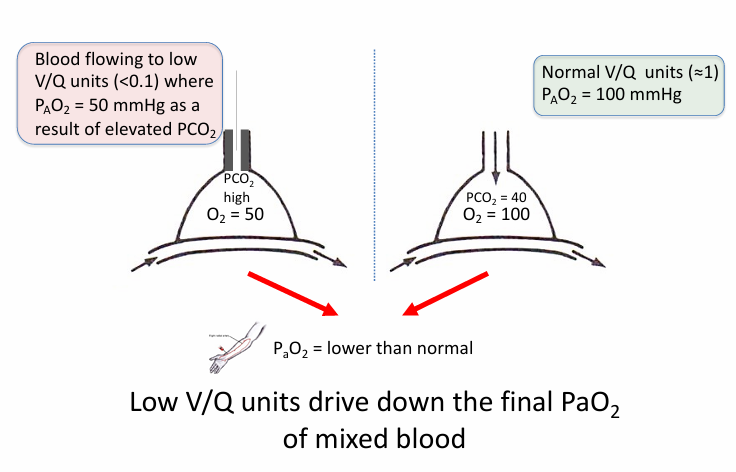
-V/Q mismatch lower the final PaO2 of the mixed blood (blood from affected low V/Q units + normal units)
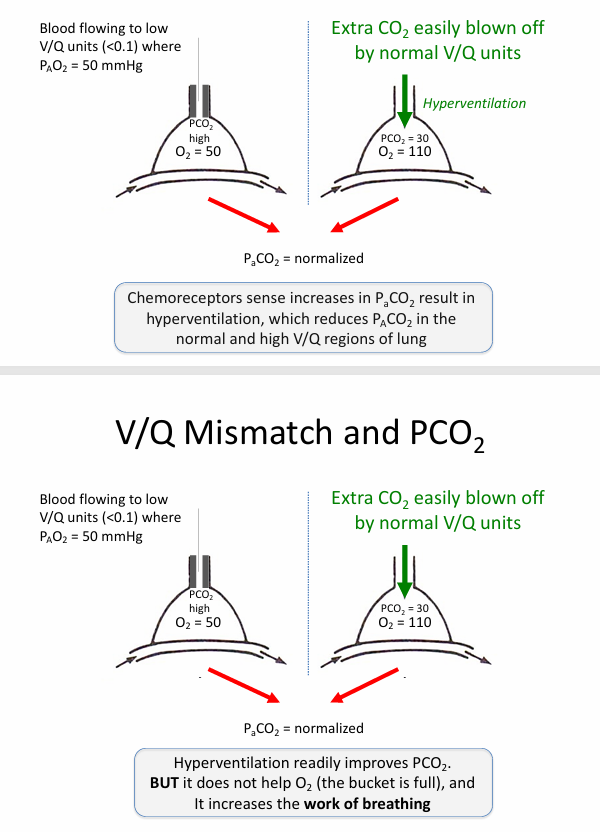
-V/Q mismatch has minimal impact on serum PCO2
-calculate the expected overall PAO2 for the patient based on the measured serum PCO2, and in the absence of a hypoventilation problem, it should be normal (or high, if there is hyperventilation)
-calculated PAO2 is normal or high; measured PaO2 is low
-V/Q mismatch increases the A-a gradient
abnormal low V/Q units in diseased lung
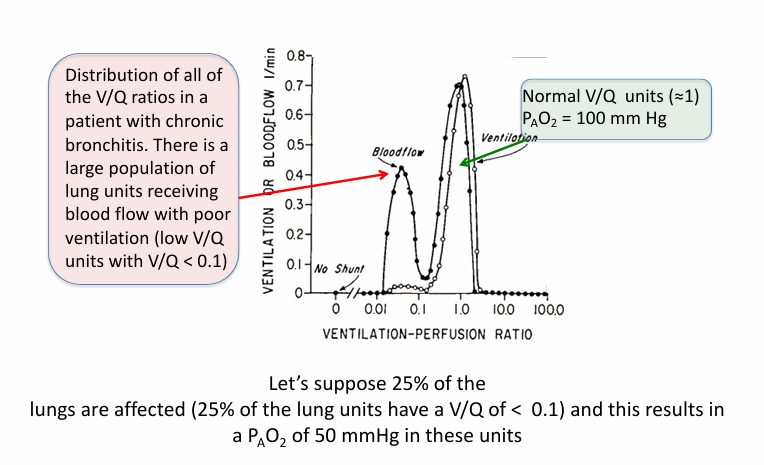
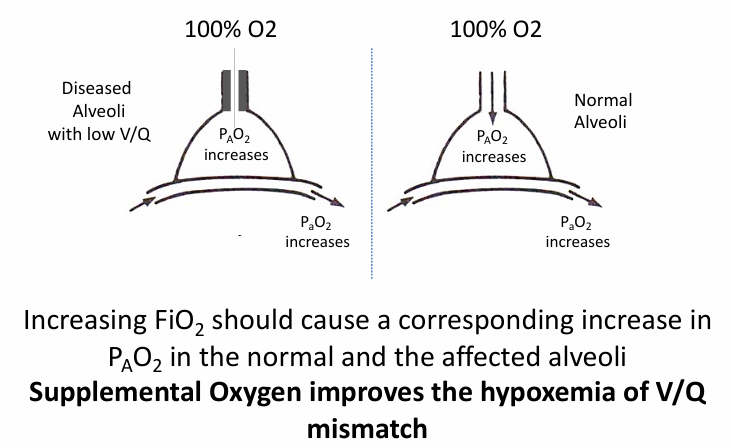
V/Q mismatch
V/Q mismatch and PCO2
hyperventilation
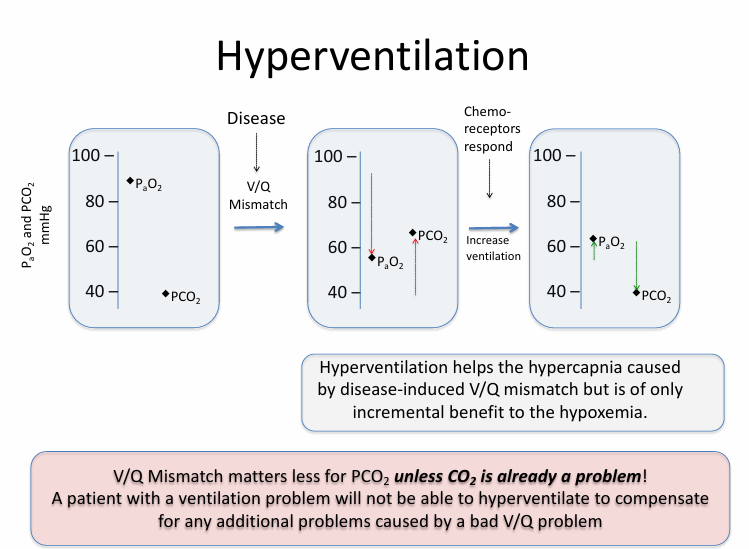
hyperventilation fails in
-severe disease
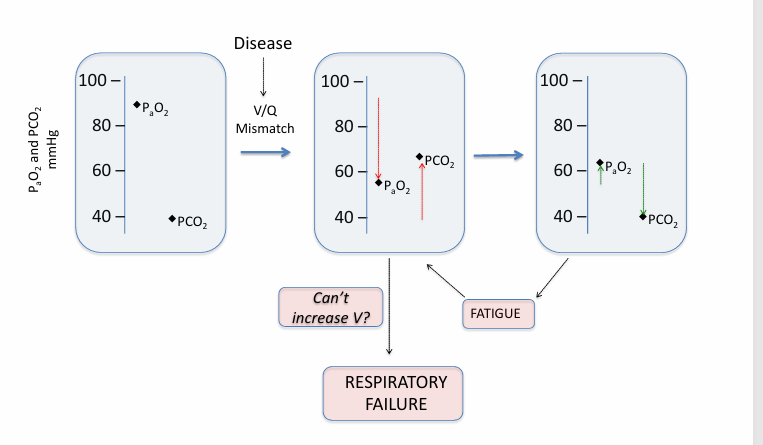
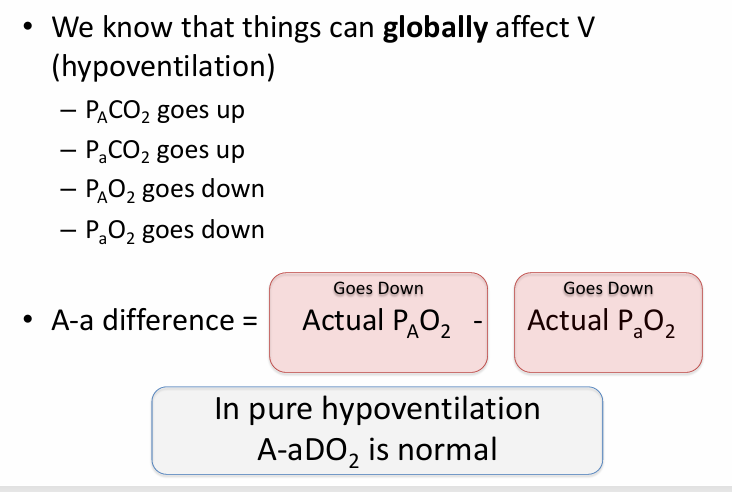
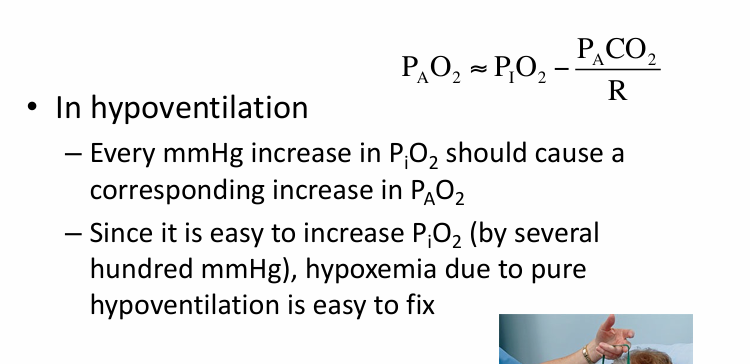
pure hypoventilation v V/Q mismatch
not all V/Q mismatch results in
-hypoxemia
extremes of V/Q mismatch
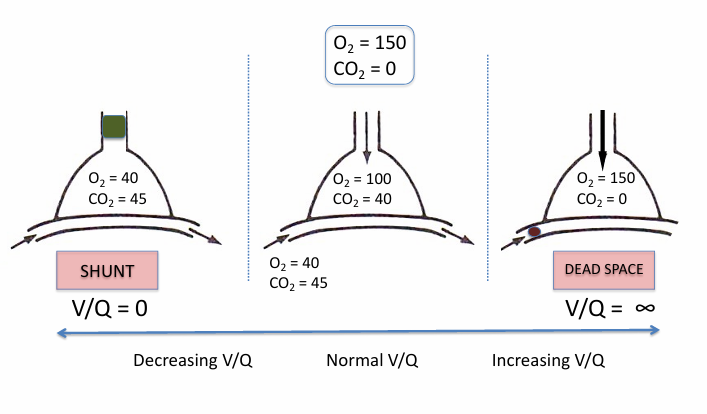
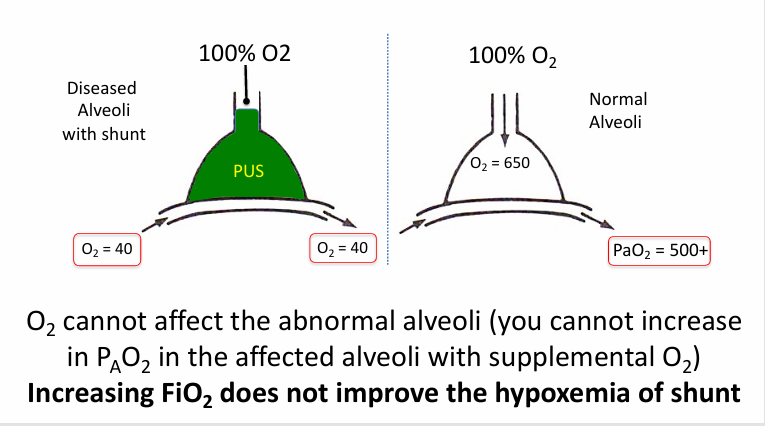
shunt
-an extreme of low V/Q mismatch when V/Q = 0
-shunt: flow of deoxygenated blood that never picks up fresh O2 and is mixed back into the oxygenated blood pool
-shunt can be in heart or lungs
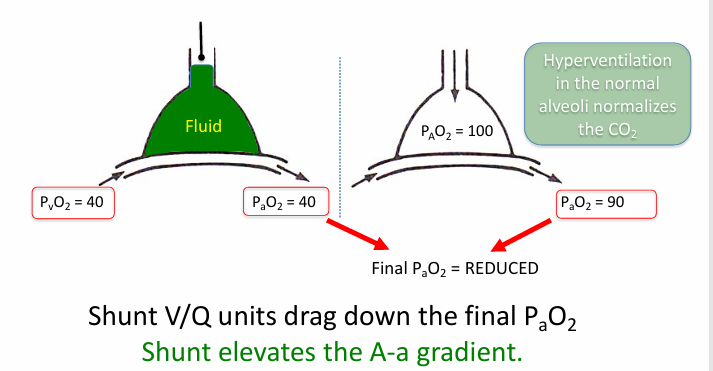
shunt in pneumonia
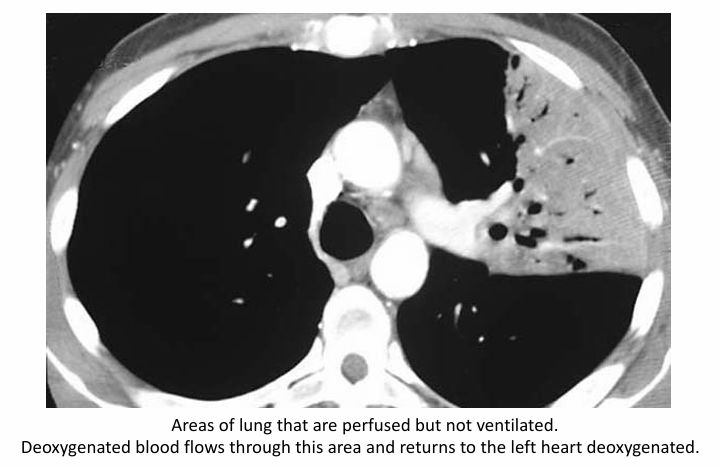
hypoxemic vasoconstriction
-underventilated alveoli have higher PCO2 and therefore lower PAO2; areas of shunt have no O2
-causes a reflex vasoconstriction of the vessels to these alveoli, therefore reducing Q to match the lowered V
-can mitigate the contribution of low V/Q units to the total pool of oxygenated blood
-compensation can be lost in disease states
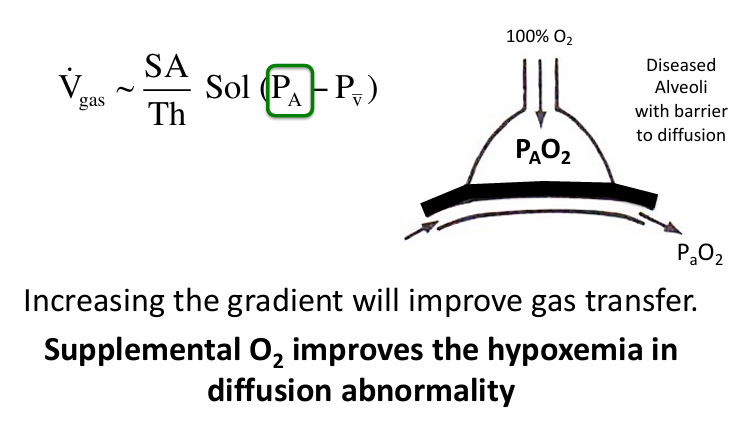
other causes of hypoxemia- diffusion abnormality
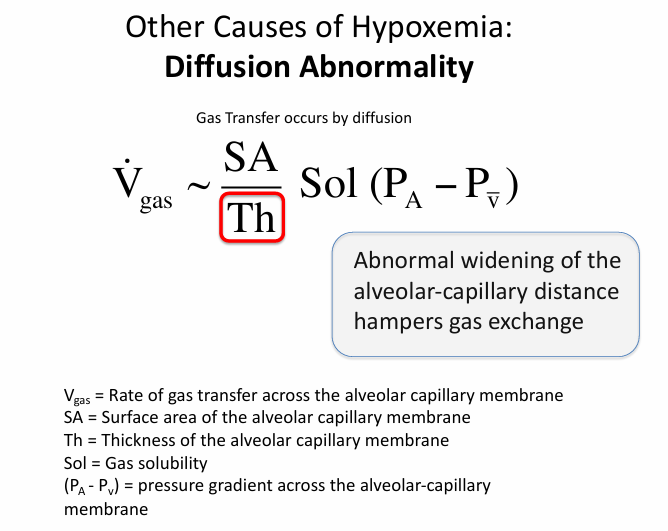
diffusion abnormality
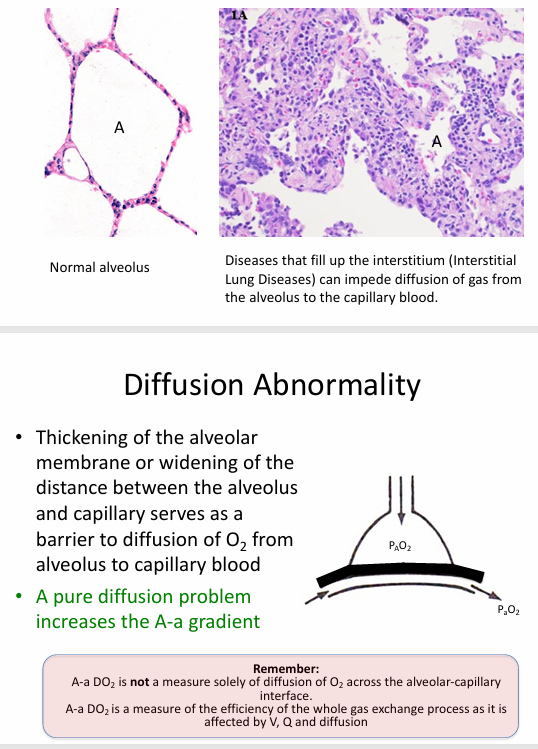
diffusion abnormality causing hypoxemia
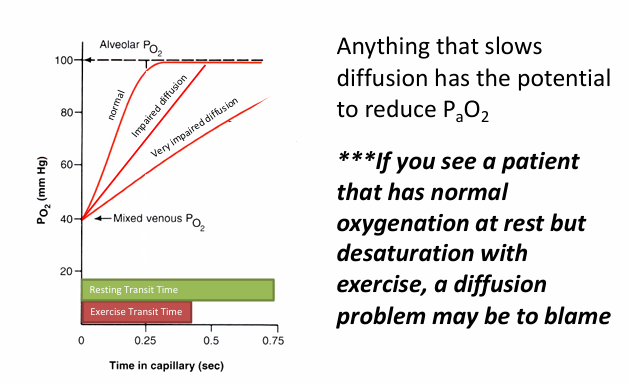
diffusion problem with exercise
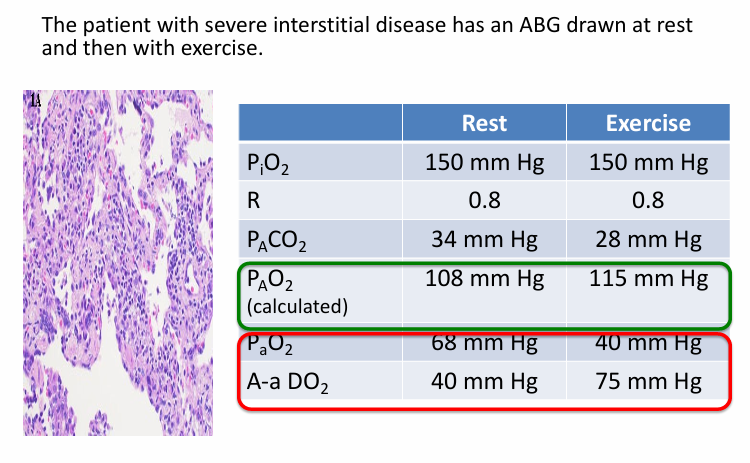
low PiO2 causing hypoxemia
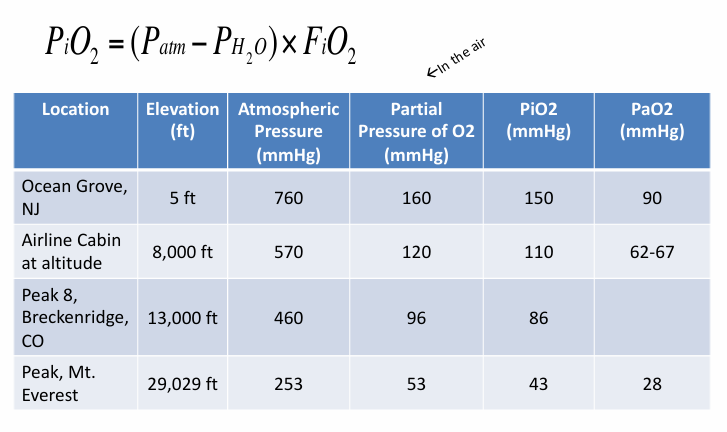
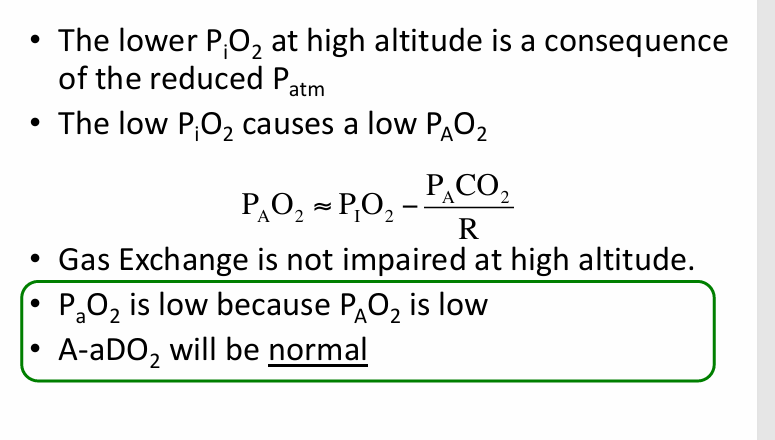
hypoxemia at altitude
supplemental O2
supplemental O2 in V/Q mismatch
supplemental O2 and diffusion abnormality
supplemental O2 in shunt
measuring gas exchange
-A-a gradient
-ratio of PaO2 to FiO2 (“P/F” ratio)
-exercise testing
-diffusing capacity: performed in the PFT lab
5 causes of hypoxemia
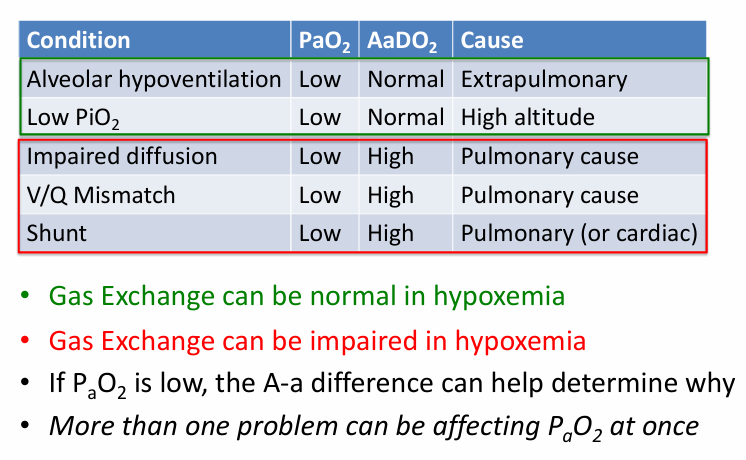
A-a gradient- higher than expected
elastance
-measure of the tendency of the lungs to recoil after removal of a distending force (inspiration)
-measure of the amount of work needed to expand the lungs
-elastic properties of the lung are due to the presence of abundant elastin fibers in the extracellular matrix
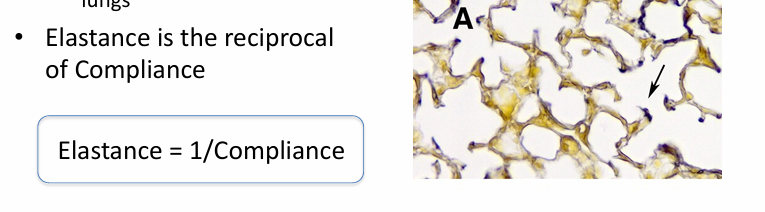
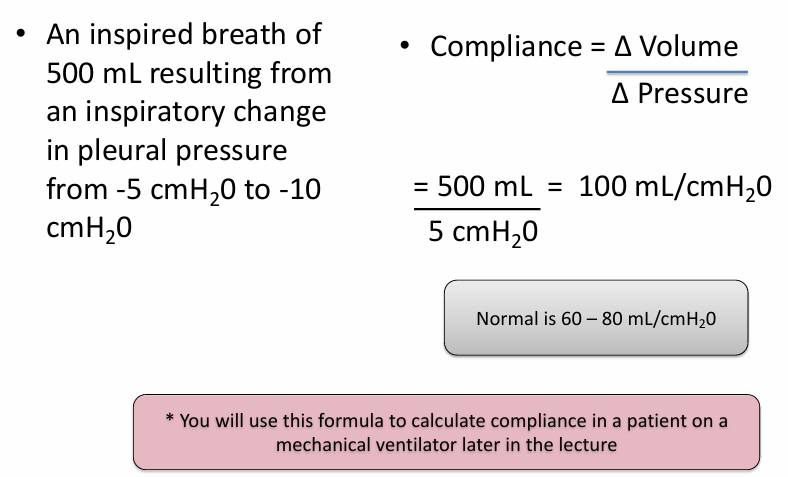
compliance
-measure of the ease of expansion of the lungs (or lungs and chest wall)
-reciprocal of elastance
-lungs can have increased compliance (emphysema) or decreased compliance (fibrosis, pulmonary edema)
-loss of compliance = “stiff lungs”
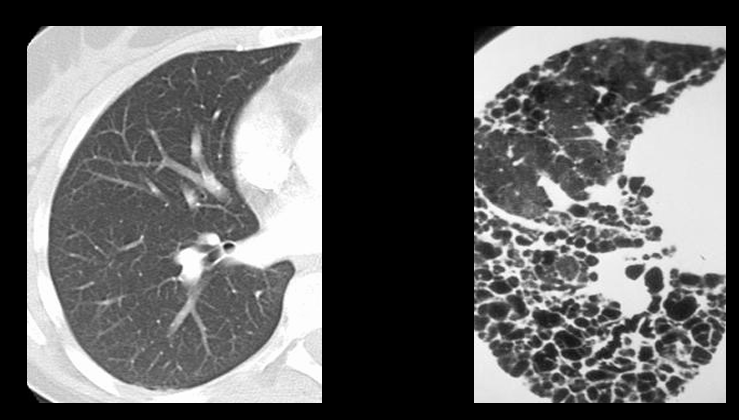
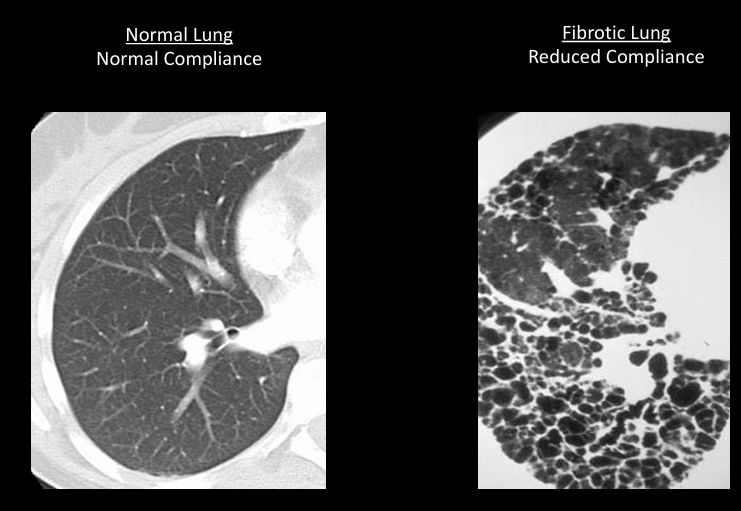
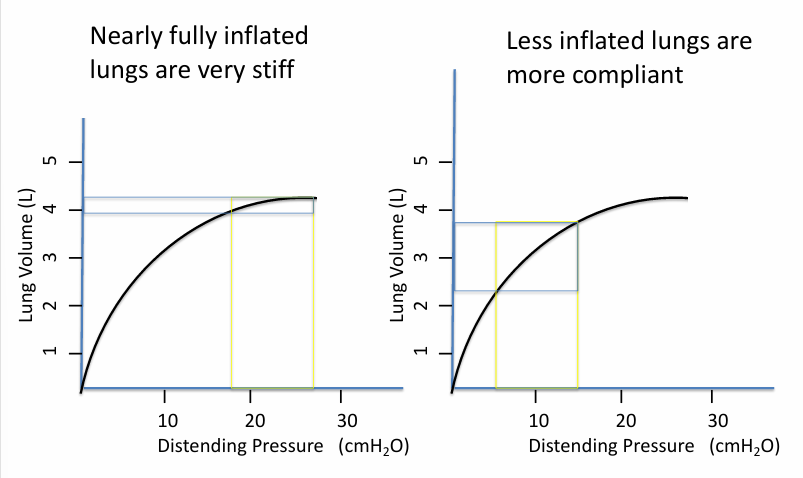
pressure/volume curve of lung inflation
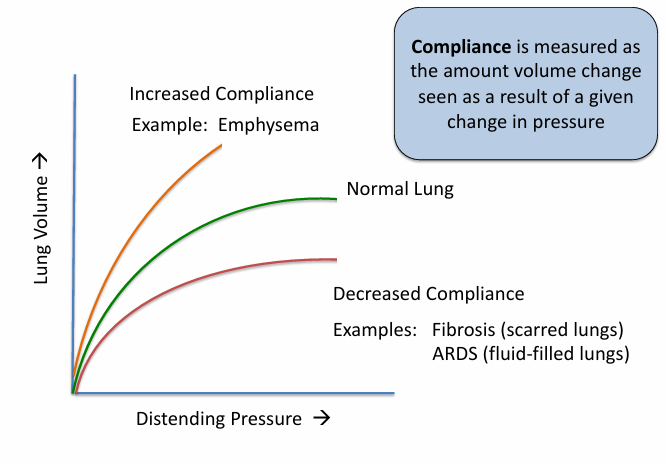
measuring lung compliance
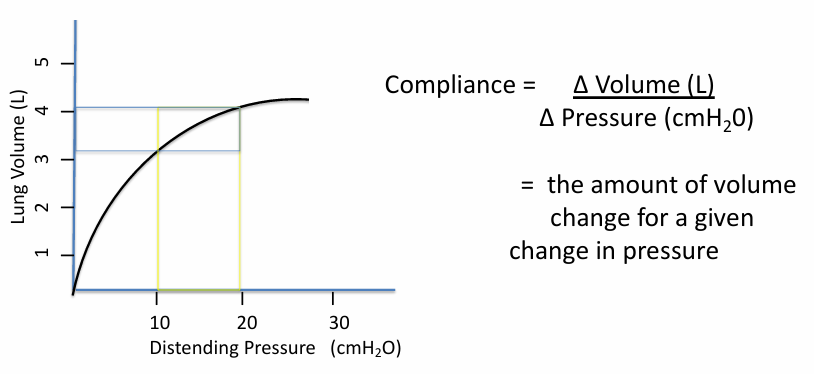
lung compliance is different depending on
-where you are on the curve (i.e. how inflated the lungs are)
calculating respiratory compliance
inflation properties of the lungs
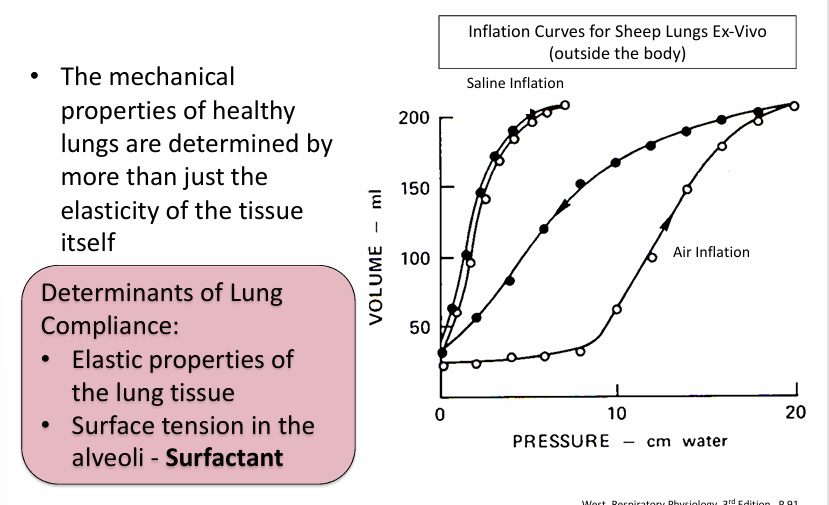
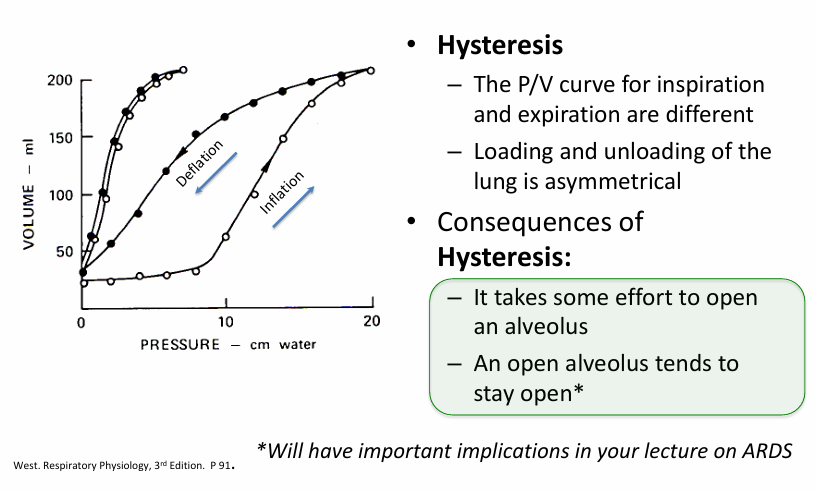
inflation properties of the lung- surfactant
-fluid that lines the alveolar surface & lowers surface tension in a way that helps to keep alveoli open
surfactant is the reason we see
-hysteresis
total compliance of the respiratory system
-lungs and chest wall together in a breathing patient
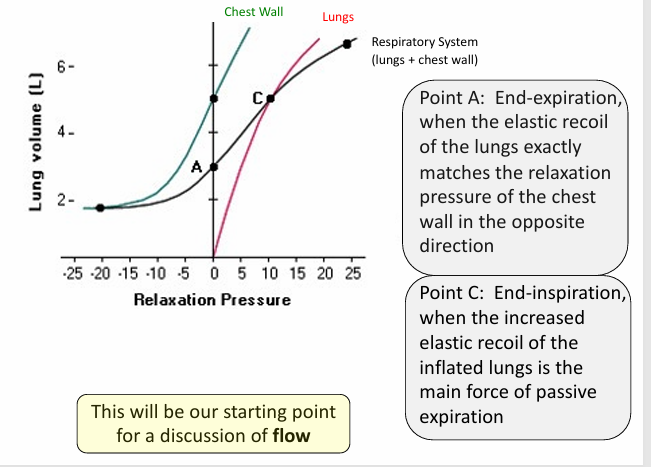
total compliance of the respiratory system- lung volume v relaxation pressure
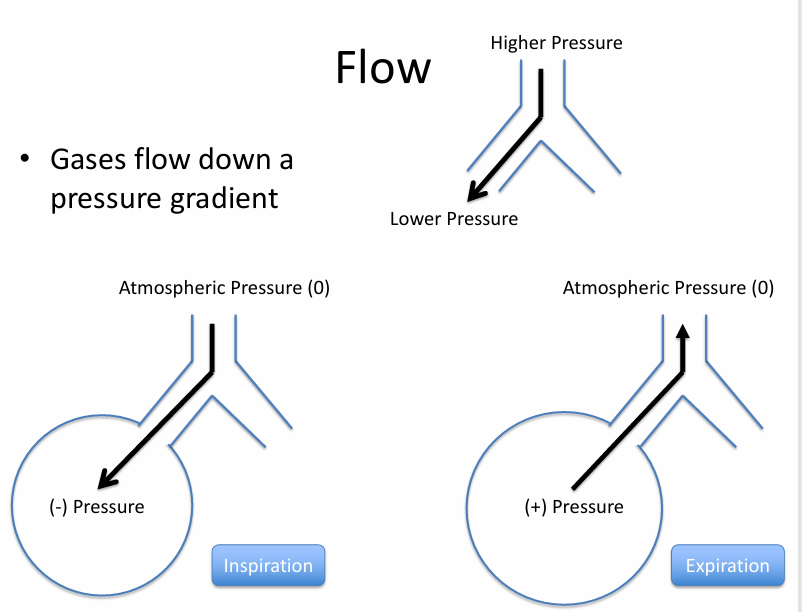
flow
-no flow at pressure equilibrium- normal end inspiration & expiration
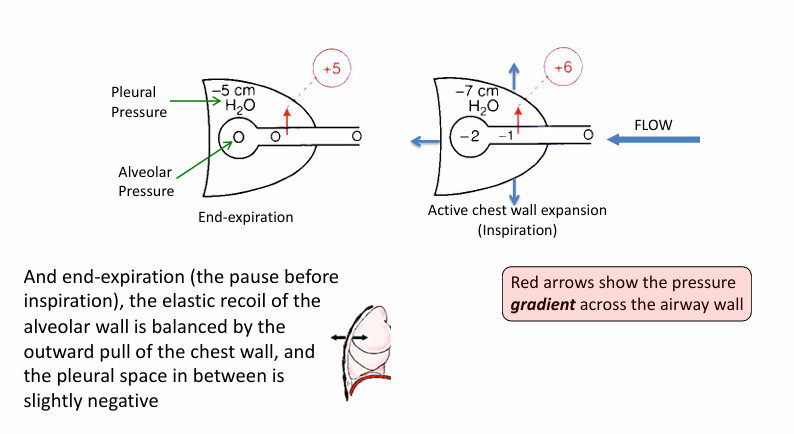
chest expansion generates
-a gradient for inspiratory flow
patterns of flow in the airways
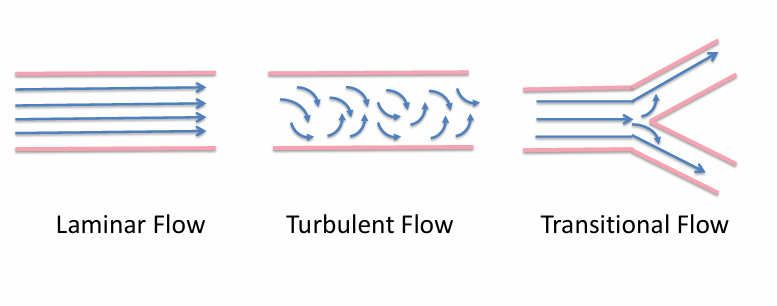
airway resistance during laminar flow
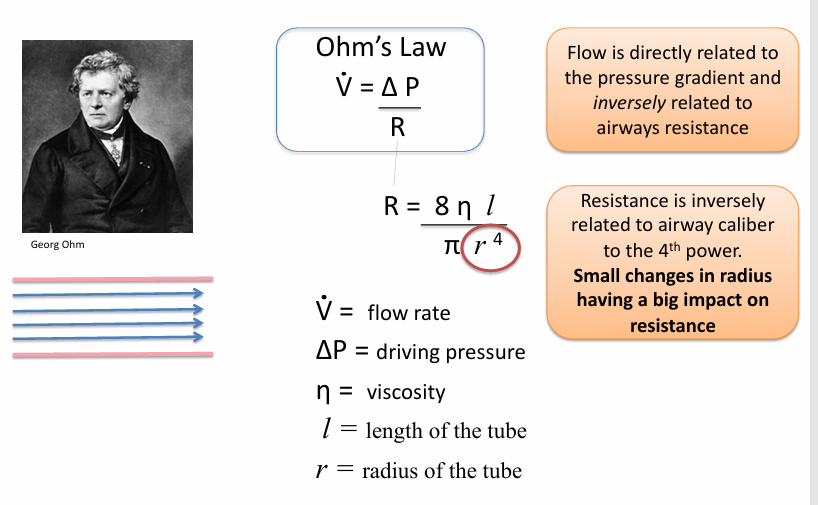
obstruction
-increases resistance to flow
-any reduction in the lumen of an airway will increase resistance to flow exponentially (r^4)
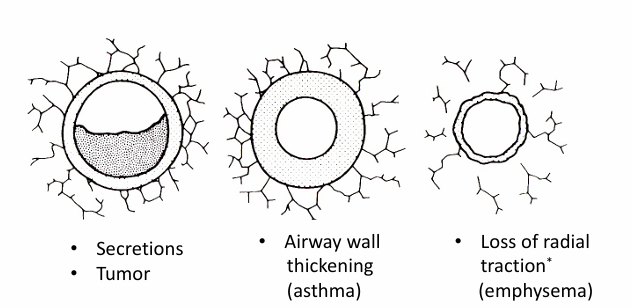
increased airway resistance increases
-the work of breathing
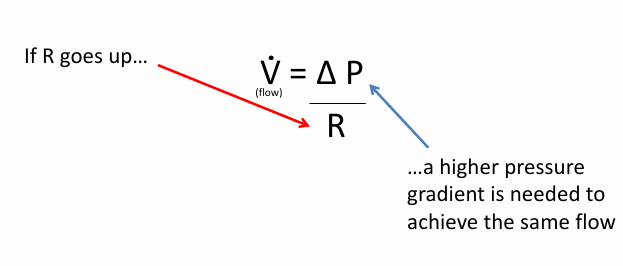
forced expiration leads to
-airway closure at the equal pressure point
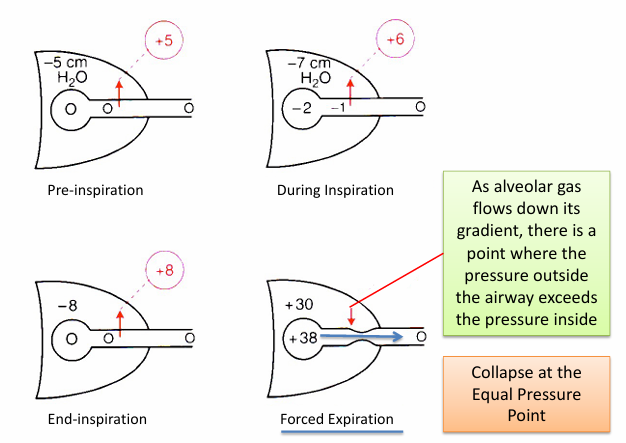
forced expiration and the equal pressure point
-in diseases that increase airway resistance: friction loss is greater, pressure drop is steeper, EPP falls further upstream
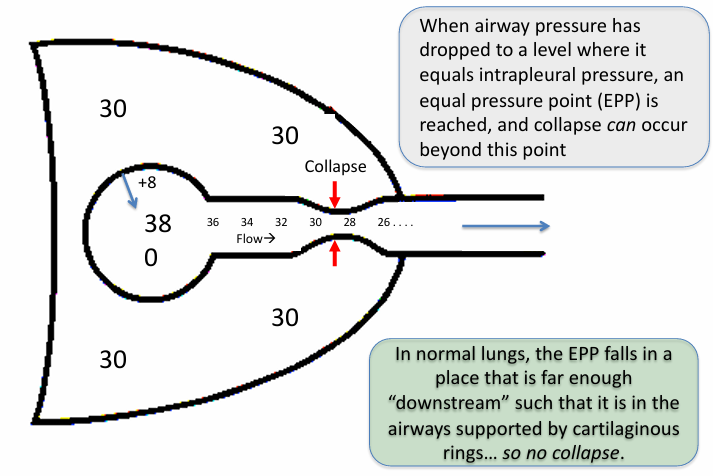
forced expiration & equal pressure point in normal lungs & airway obstruction
-normal lungs: airway resistance is low and alveolar driving pressure (lung recoil pressure) is high; EPP will be reached in cartilaginous airways → no collapse
-airway obstruction: resistance to airflow is higher and pressure drop will be much steeper; EPP is further upstream (toward the alveoli) in the thin-walled bronchioles → airway collapse
-causes the typical depression in the flow-volume curve seen in an OVD
pulmonary function tests- standard PFTs
-spirometry
-lung volume testing (plethysmography, N2 washout, helium dilution)
-diffusion capacity
additional PFTs that are sometimes performed
-respiratory muscle pressure testing
-bronchial challenge testing
-impulse oscillometry
-blood gas testing
-six-minute walk test
-pulse oximetry
how are “normal” PFT values determined?
-PFT interpretation requires comparison of a patient’s values with appropriate reference equation values: current standard- LLN, old standard- % predicted
-global lung function initiative (GLI): utilized 160,000 data points from 33 countries to develop most widely-accepted reference equations- Caucasians, African Americans, Northeast Asians, Southeast Asians, other
-reference equation parameters: age, sex, height, race/ethnicity
PFT race corrections
-rested on an erroneous scientific foundation
-decreased lung function in mid/late adulthood is a consequence of: inadequate development in utero and childhood, accelerated decline
-trajectories of lung growth vary in children exposed to different amounts of air pollution
-maternal nutrition and tobacco smoke exposure have a similar impact on fetal lung development
-Samuel Cartwright and others who developed race-based PFT corrections did not account for these social determinants of lung function
PFT race corrections have consequences
-patients requiring resectional surgery for lung cancer undergo PFTs to assess candidacy
-pulmonary fxn is often tested before employment or to assess insurability: prospective and current firefighters, those seeking enlistment in armed services
-black individuals are more likely to be undertreated and have worse outcomes for lung diseases than whites, in part due to misclassification of lung function
spirometry
-simplest, most common pulmonary function test
-assesses flow and volume of forced air during inspiration and expiration
-results can be distorted by inadequate effort or lack of cooperation with test administrator
-results are reported as volume vs time or flow vs volume: absolute values (L or L/s), % predicted, Z score (for LLN)
what we measure with spirometry
-FVC: forced vital capacity (total expired breath volume)
-FEV1: breath volume expired during 1st second
-FEF25-75%: mean flow rate of expiration during middle ½ of breath
-PEF: peak expiratory flow rate
-FET: forced expiratory time
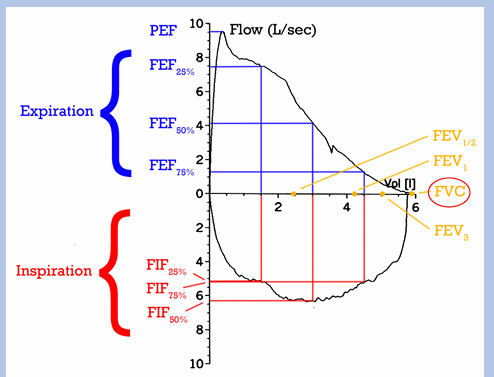
what we measure with spirometry- ratios
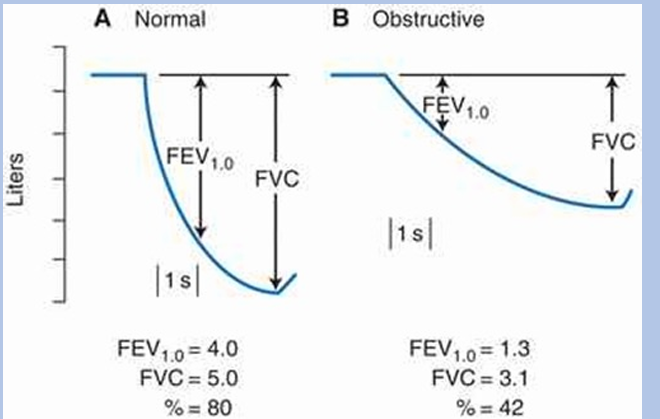
-FEV1/FVC ratio- typical value is 80%
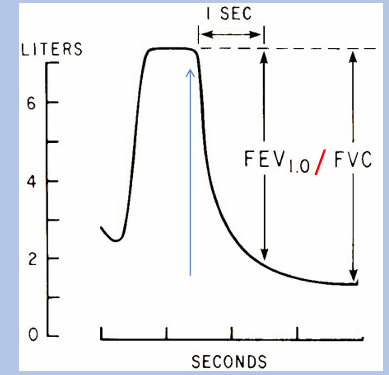
obstructive airway diseases
-characterized by a reduced airflow during expiration, leading to a decline in FEV1/FVC ratio → “obstructive ventilatory defect”: obstruction classically defined as FEV1/FVC ratio < 0.70, today it’s typically defined as FEV1/FVC ratio < LLN (LLN = lower limit of normal; better accounts for variation in FEV1/FVC with age, sex, and height)
-obstructive airway diseases: asthma, COPD, cystic fibrosis, bronchiectasis, upper airway disorders, bronchiolitis
obstructive airway diseases FEV & FVC
-obstruction is consequence of airway compression during expiration
-effect is exacerbated during a forceful expiratory maneuver done during spirometry
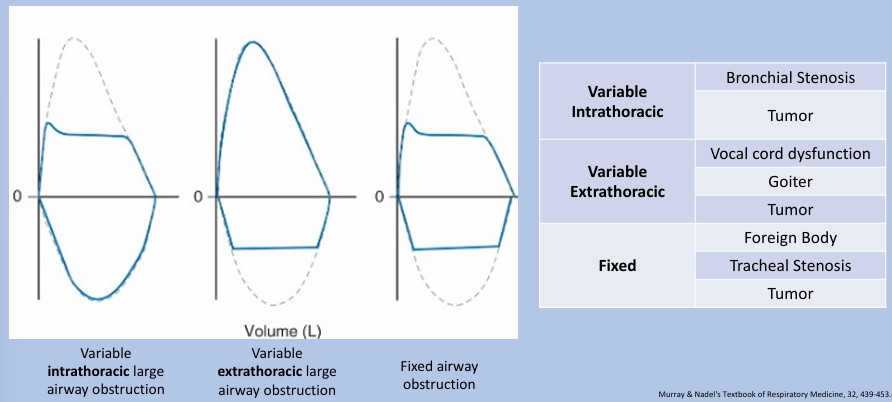
flow-volume loop patterns of large airway obstruction
lung volume testing
-pulmonary function tests focused on measuring different lung volumes (not flows)
-unlike spirometry, allows for measurement of volume remaining after forced exhalation (residual volume), and therefore total lung capacity
-multiple methods of lung volume testing: plethysmography (most accurate and commonly used method), nitrogen washout, helium dilution
plethysmography
-utilizes Boyle’s law: P1V1 = P2V2
-patient sits in “body box” with mouth connected to tube, pressure and volume within box is monitored
-patient pants through tube which allows box pressure/volume changes to be measured

nitrogen washout
-patient breathes in 100% oxygen and exhales into nitrogen detector
-after successive breaths, expired % nitrogen goes to zero, at which point FRC can be calculated from expired nitrogen volume
-less accurate than plethysmography due to: mouthpiece leaks, airway obstruction (prolongs nitrogen expiration), bullae (do not participate in breath)
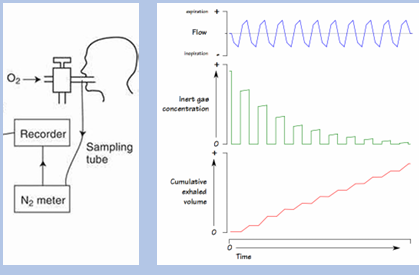
helium dilution
-similar concept
-patient breathes in/out through tube connected to helium reservoir
-once helium concentrations equilibrate, FRC can be solved for
-like N2 washout, also less accurate than plethysmography due to similar issues
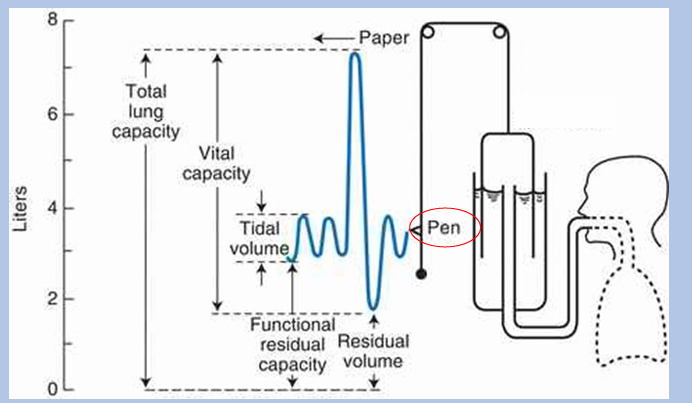
lung volumes and capacities
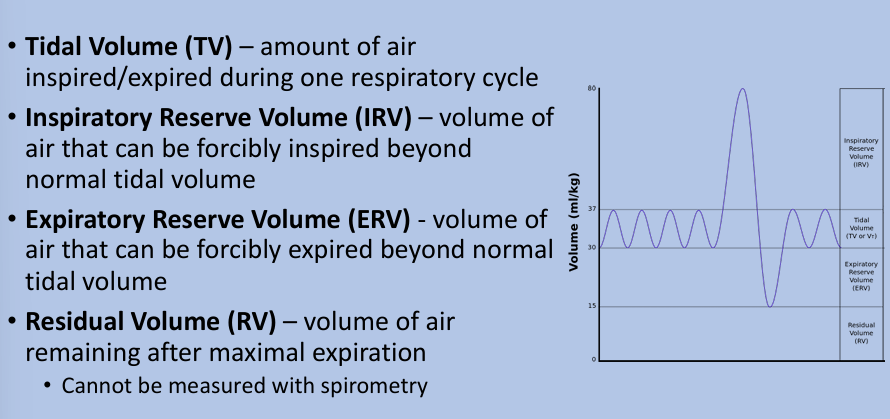
total lung capacity, vital capacity, functional residual capacity
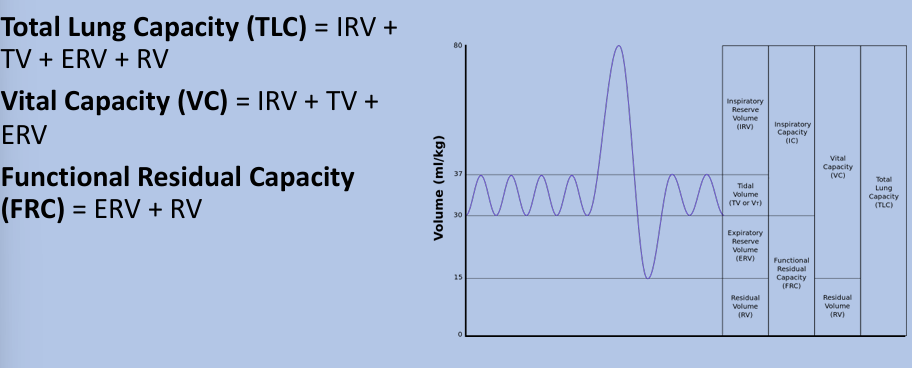
lung volume tracings
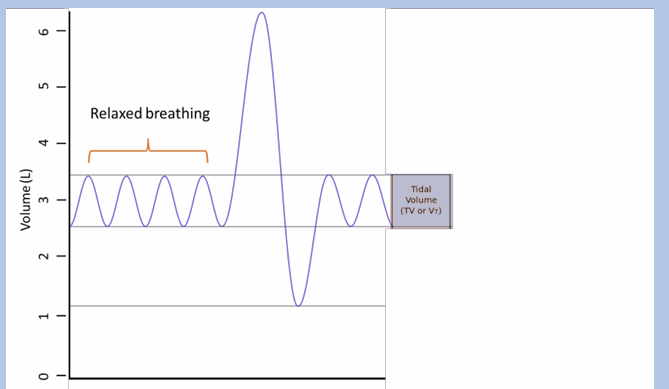
tidal volume
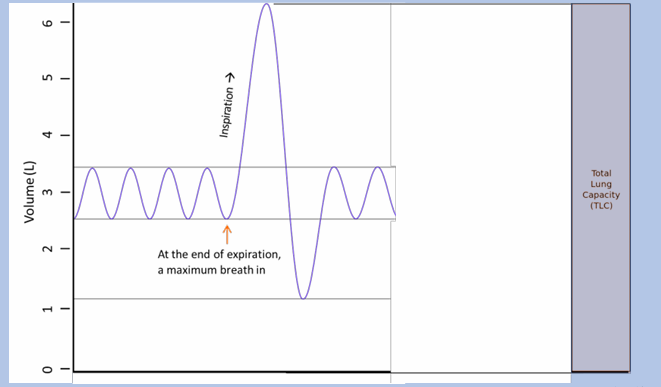
total lung capacity
functional residual capacity
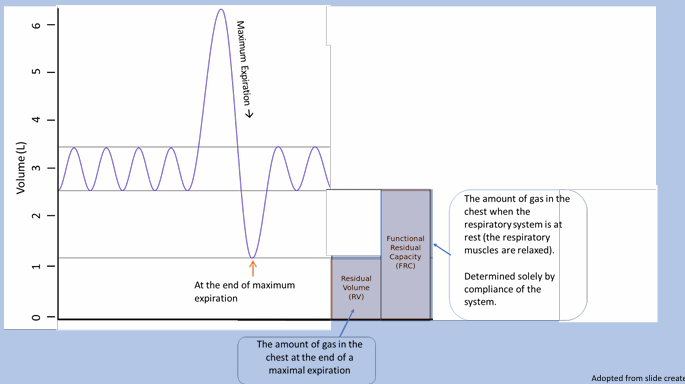
restrictive lung diseases
-defined as TLC less than lower limit of normal (classically 80% predicted): can be suggested on spirometry with proportional reduction in FEV1 and FVC, but lung volume testing is only means of confirming diagnosis
causes of restrictive lung diseases
-decreased lung compliance 2/2 abnormal lung parenchyma: diffuse pulmonary lung diseases (“interstitial lung diseases”, such as idiopathic pulmonary fibrosis)
-decreased chest wall compliance: chest wall disease (scoliosis, pectus excavatum, obesity), pleural disease
-respiratory muscle weakness
-lung collapse, resection
hyperinflation
-defined as TLC greater than upper limit of normal classically 120% predicted)
-usually driven by a relative/absolute increase in residual volume
-causes: gas trapping due to obstruction (severe asthma, COPD), increased lung compliance (emphysema)
why can’t restriction be diagnosed with spirometry?
-low FVC on spirometry can be an indicator of a low TLC on lung volume testing
-can also be an indicator of gas trapping: RV is increased, VC is reduced, and TLC is either normal or increased
-therefore, restrictive lung disease can only be diagnosed with lung volume testing!
gas trapping
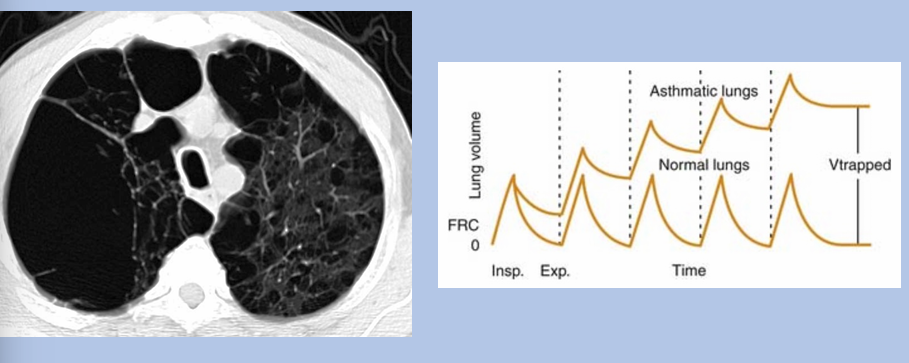
can obstruction and restriction coexist?
-yes!
-obstruction is an airflow (airway) problem: diagnosed via spirometry
-restriction is a lung volume problem: diagnosed via lung volume testing
-therefore, presence of both simultaneously is possible
lymphangioleiomyomatosis (LAM)
-rare disease characterized by abnormal growth of smooth muscle cells leading to formation of innumerable lung cysts
-cysts compress distal airways → obstruction
-thoracic duct compressed → chylous pleural effusion → restriction
diffusion capacity
-test which assess the ability of lungs to transfer oxygen from inspired air into the bloodstream
-carbon monoxide is the gas typically used in diffusion capacity testing, not O2
gases transfer via diffusion
-ratio of alveolar membrane surface area to its thickness is diffusion capacity
-higher diffusion capacity = greater ability to diffuse gas
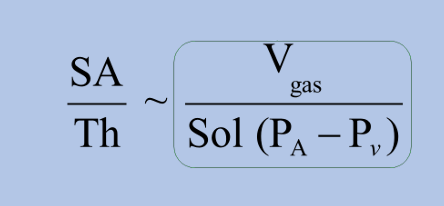
why do we use CO for diffusion testing?
-to assess for alveolar diffusion capacity, need a gas with the following properties:
1) gas transfer is limited by diffusion: some gases like CO2 diffuse so quickly that their transfer is perfusion-limited
2) gas is absent in the blood: one reason why we don’t use O2, as the PaO2 would need to be known to calculate diffusion capacity
3) gas is not toxic (at low doses)
-carbon monoxide fits all criteria, so standard diffusion assessment is diffusion capacity of carbon monoxide (DLCO)
DLCO
-DLCO assesses the ability of alveoli to transfer CO
-VCO calculated by comparing what amount of CO a patient inhales vs exhales
-PaCO is alveolar pressure of CO, which can be calculated based on CO content of expired air
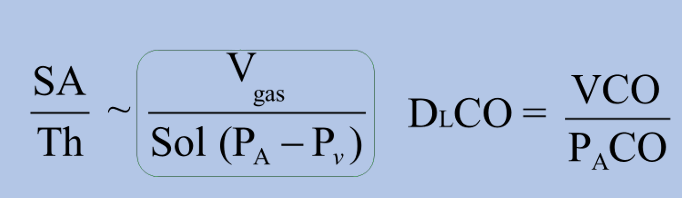
causes of DLCO reduction
-conditions affecting alveoli: emphysema, interstitial lung diseases, pulmonary edema, pneumonia
-conditions decreasing number or size of alveoli: emphysema, severe restriction, lung resection
-conditions decreasing gas carrying capacity: anemia, carboxyhemoglobinemia (from poisoning or smoking)
-conditions decreasing alveolar capillary blood flow: pulmonary hypertension, congestive heart failure
conclusions
