3.1.11 Electrode Potentials and Electrochemical cells
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
What is a half cell?
A metal dipped into a solution containing its own ions, which establishes an equilibrium between the metal ions and the metal atoms.
Write a half equation for a zinc half cell.
Zn²⁺(aq) + 2e⁻ → Zn(s)
What is the salt bridge made up of?
A piece of filter paper soaked in a solution of potassium nitrate (or chloride).
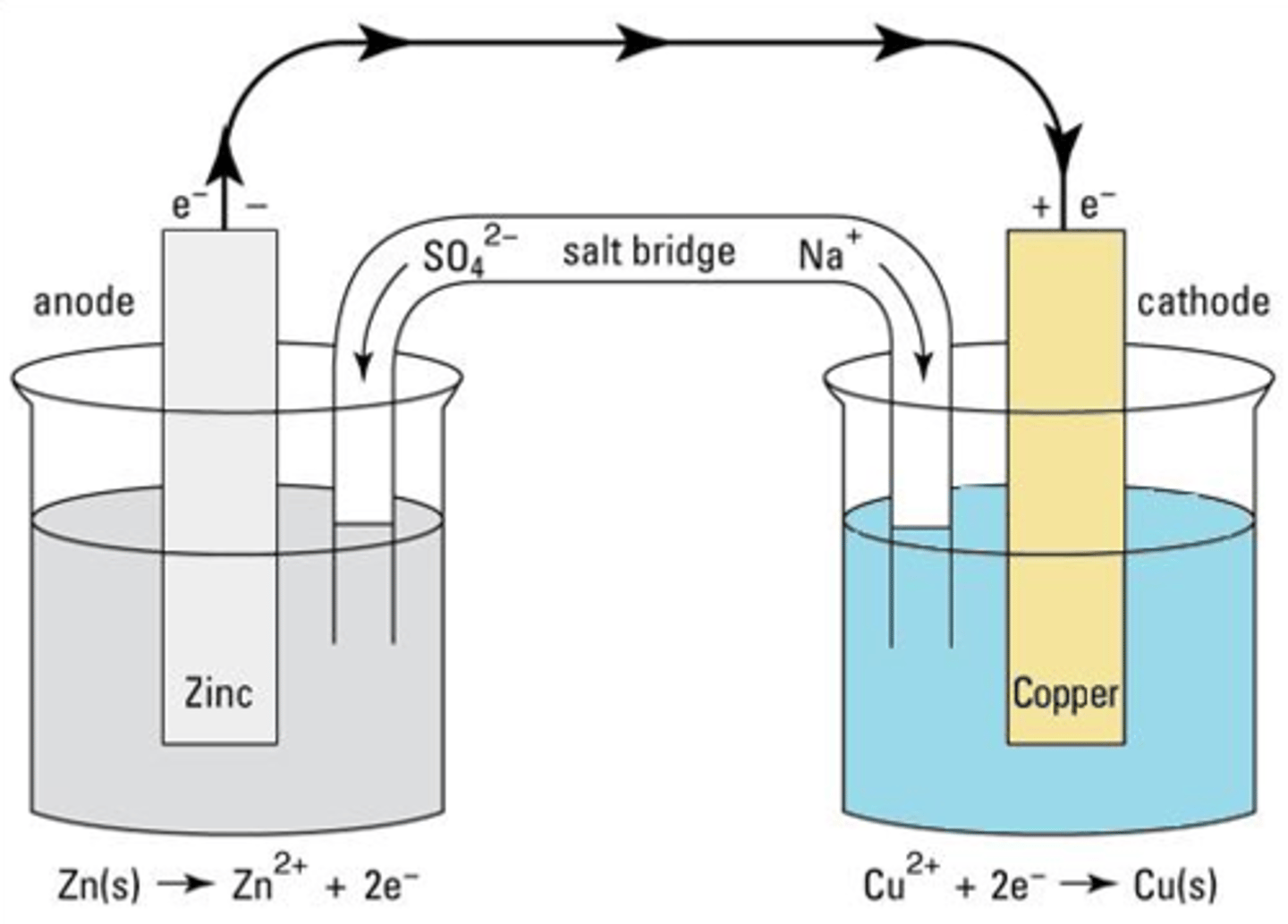
Draw a cell, with zinc metal on the left and copper metal on the right.
Two beakers connected by a salt bridge.
Zinc metal immersed in the left beaker, with water and Zn²⁺ ions drawn. Conc of Zn²⁺ noted as 1moldm⁻³.
Copper metal immersed in the right beaker, with water and Cu²⁺ ions drawn. Conc of Cu²⁺ noted as 1moldm⁻³.
Wire connecting zinc and copper, with a voltmeter in the middle of the wire (NOTE: this will stop electrons from flowing).
Explain the purpose of the salt bridge.
The salt bridge has mobile ions that complete the circuit. Potassium nitrate is used to ensure that there is no precipitation since nitrates are soluble.
For a zinc-copper cell, which metal would lose mass and which would gain mass?
Zinc would lose mass, since it is a stronger reducing agent.
An electrode where oxidation occurs is the ________.
The electrode where reduction occurs is the ________.
Anode (negative)
Cathode (positive)
Describe what is happening in a zinc-copper cell.
The zinc forms the negative electrode and the copper forms the positive electrode.
Zinc is oxidised and loses electrons, which flow from the zinc metal to the copper metal. Copper ions gain the electrons, so are reduced to form solid coper.
Copper forms on the copper electrode and so increases the mass.
Some zinc forms zinc ions and so decreases the mass of the zinc electrode.
This shows that zinc is more likely to form its ions than copper (so the equilibrium lies closer to the left).
Draw a Daniell cell. Write the IUPAC conventional representation of this cell.
Zn(s)│Zn²⁺(aq) || Cu²⁺(aq)│Cu(s) EMF = +1.10V
Explain why Cu²⁺ is a better oxidising agent than Zn²⁺.
Cu²⁺ has a more positive standard electrode potential.
In order to measure the voltage/potential difference of this cell, a voltmeter is attached to the wire in series with the circuit. Explain the effect this has.
The high-resistance voltmeter prevents any electrons from flowing through the circuit, maintaining the concentration of the ions in solution by not allowing current to flow through.
The voltmeter can still measure the potential difference of the electrons trying to flow.
This gives the voltage between half cells, not for one half cell.
Define standard electrode potential (E°)
The electrode potential of a standard electrode with ion concentration 1.00moldm⁻³ at 298K connected to a standard hydrogen electrode with H⁺ ion concentration of 1.00moldm⁻³, with 100kPa H₂ gas at 298K, using a high-resistance voltmeter and a salt bridge.
Draw a diagram to represent the standard hydrogen electrode. Write a half equation for the reduction of H⁺ ions.
(Changes to diagram:
"1bar" replaced by 100kPa
"Dilute sulphuric acid" removed.)
2H⁺(aq) + 2e⁻ → H₂(g)
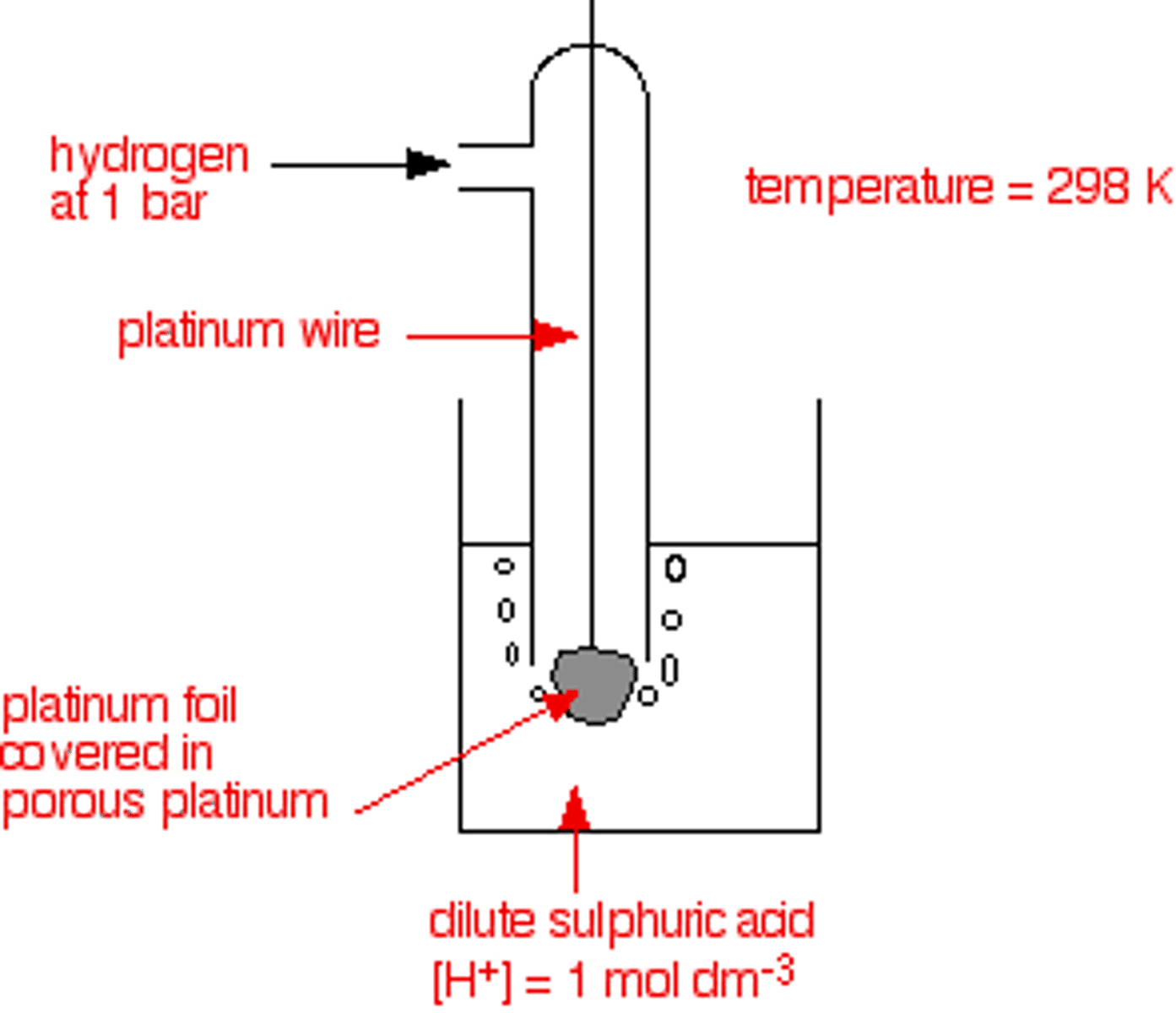
Why is platinum used?
It is a good conductor of electricity and is inert. Porous platinum gives a larger surface area for reaction.
Draw a diagram to represent how the standard electrode potential of copper would be measured.
Explain the value generated, using half equations.
(voltmeter in middle of wire)
The value generated would be positive, since Cu²⁺ is a stronger oxidising agent than H⁺, so electrons are trying to flow from the hydrogen electrode to the copper electrode.
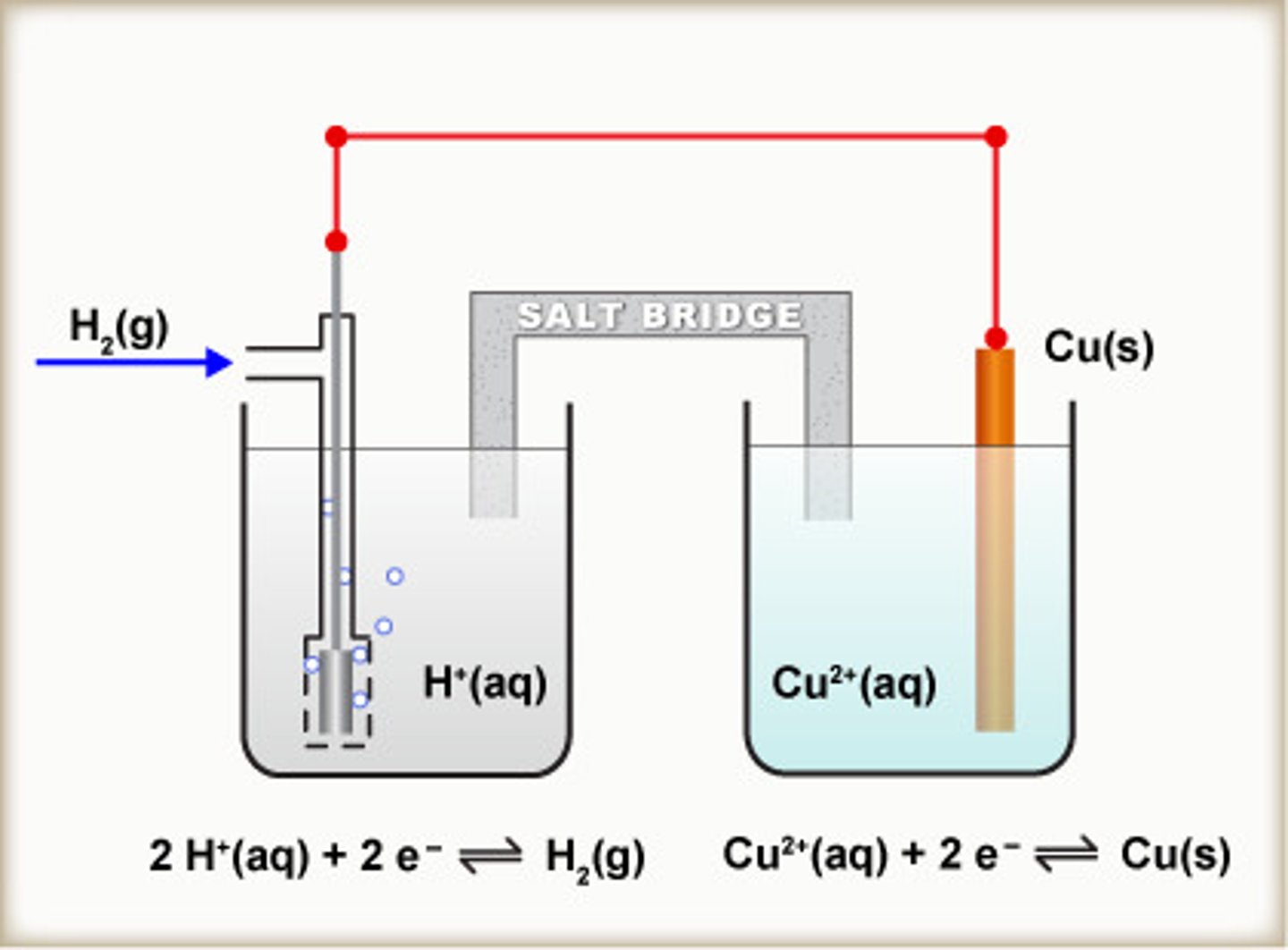
Write the IUPAC conventional representation of a standard hydrogen electrode and copper electrode.
Pt(s)│H₂(g)│H⁺(aq) || Cu²⁺(aq)│Cu(s) EMF = +0.34V
Explain why the standard electrode potential of zinc is negative.
Zn is a stronger reducing agent than H₂, so electrons are trying to flow from the zinc electrode to the hydrogen electrode, giving a negative value.
Explain how you would measure the standard electrode potential of a solution of two ions.
Make a half cell, using a platinum electrode, immersed in a solution containing equal concentrations of both ions. Connect this to a standard hydrogen electrode using a salt bridge and wire, with a high-voltage voltmeter.
Write the IUPAC conventional representation to show the standard electrode potential of a Fe²⁺ and Fe³⁺ solution. State the concentration of these ions in solution.
Pt(s)│H₂(g)│H⁺(aq) || Fe³⁺(aq) , Fe²⁺(aq)│Pt(s) EMF +0.77V
What is meant by the electromotive force?
The overall voltage measured on a cell.
Why may an ammeter be used instead of a voltmeter?
To show the direction of electron flow in the cell.
The more positive the electrode potential, the more ________ the ________ reaction is.
feasible, reduction
If the EMF is ________, the redox reaction is feasible. If it is ________, the reaction is not feasible.
positive, negative
Which species is the:
strongest oxidising agent,
strongest reducing agent,
weakest oxidising agent,
weakest reducing agent?
F₂
Li
Li⁺
F⁻
Given that E⁰ value for Cl₂ + 2e⁻ → 2Cl⁻ = +1.36V and the E⁰ value for Br₂ + 2e⁻ → 2Br⁻ = +1.07V,
Explain why the following reaction is not feasible:
2KCl + Br₂ → 2KBr + Cl₂
The following reactions are occurring:
Br₂ + 2e⁻ → 2Br⁻ (reduction)
2Cl⁻ → Cl₂ + 2e⁻ (oxidation)
Therefore the EMF = 1.07 - 1.36 = -0.29V
EMF is negative and so not feasible.
With conventional cell representation, what does one vertical line represent?
A phase boundary.
What is the equation for EMF?
EMF = E⁰(reduction) - E⁰(oxidation)
(or RHS - LHS)
Electrochemical cells can be used as a ________ source of ________ energy.
commercial, electrical
What is the difference between a primary cell and a secondary cell?
A primary cell is non-rechargeable (irreversible), whereas a secondary cell is rechargeable.
How do you balance redox equations in alkaline conditions?
Add as many hydroxide groups to both sides as there are hydrogen ions. This forms water on one side and hydroxide on the other.
Draw a non-rechargeable cell. Write the half equations for this redox reaction, and so write an overall reaction for when the cell discharges.
What is the oxidation state of both manganese species?
Carbon rod (made from graphite) with positive charge at top. Surrounded by MnO₂ paste, then ZnCl₂ paste (separated by porous separator), with zinc used as the container. Cover on top.
Oxidation:
Zn(s) → Zn²⁺(aq) + 2e⁻
Reduction:
MnO₂(s) + H₂O(l) + e⁻ → MnO(OH) (aq) + OH⁻(aq)
Zn + 2MnO₂ + 2H₂O → 2MnO(OH) + 2OH⁻ + Zn²⁺
oxidation state goes from +4 to +3.
Given that
Zn²⁺(aq) + 2e⁻ → Zn = -0.76V and
MnO₂(s) + H₂O(l) + e⁻ → MnO(OH) (aq) + OH⁻(aq) = +0.74V,
work out the EMF of this non-rechargeable cell.
0.74 - - 0.76 = 1.50V
Write the half equations for a Nickel-Cadmium cell (rechargeable), and so write an overall reaction for when the cell discharges.
Oxidation:
Cd(s) + 2OH⁻(aq) → Cd(OH)₂(s) + 2e⁻
Reduction:
NiO(OH) + H₂O(l) + e⁻ → Ni(OH)₂(s) + OH⁻(aq)
Cd + 2NiO(OH) + 2H₂O → Cd(OH)₂ + 2Ni(OH)₂
What are advantages of rechargeable cells in comparison to non-rechargeable cells?
They are more environmentally advantageous since they can be reused and prevent waste.
Supplies of the metal and other reagents are not depleted as quickly and less energy is used to extract metals.
How is a cell recharged?
The reagents are regenerated by reversing the reaction. A higher voltage is run through the cell (than its E⁰).
What cell is used in cars? How is it recharged?
Lead-acid cell
It is recharged as the vehicle moves.
Write the half equations for a lead-acid cell, and so write an overall reaction for when the cell discharges.
Oxidation:
Pb(s) + HSO₄⁻(aq) → PbSO₄(s) + H⁺(aq) + 2e⁻
Reduction:
PbO₂(s) + 3H⁺(aq) + HSO₄⁻(aq) + 2e⁻ → PbSO₄(s) + 2H₂O(l)
Pb + 2HSO₄⁻ + PbO₂ + 2H⁺ → 2PbSO₄ + 2H₂O
What is an issue if a lead-acid cell is discharged for a long period of time?
The insoluble lead sulphate builds up in the cell, preventing it from being recharged.
Write the half equations for a lithium ion cell, and so write an overall reaction for when the cell discharges.
Oxidation:
Li → Li⁺ + e⁻
Reduction:
Li⁺ + CoO₂ + e⁻ → Li⁺[CoO₂]⁻
Overall equation:
Li + CoO₂ → Li⁺[CoO₂]⁻
Write the conventional cell representation for a lithium ion cell.
Li│Li⁺ || Li⁺, CoO₂│LiCoO₂│Pt
Where are lithium ion cells used?
Mobile phones, laptops.
The reactants in this electrochemical cell are absorbed into graphite powder which acts as a support medium. The ions can react in this medium without the need for a solvent.
Explain why water cannot be used.
Water would react with lithium.
What are fuel cells?
An electrical cell which converts the chemical energy of a redox reaction into electrical energy. The cell continues to function as long as the fuel and oxygen are supplied to it. Fuel cells do not need to be electrically recharged.
Write the half equations for a hydrogen fuel cell in acidic conditions, and so write an overall reaction for when the cell discharges.
Oxidation:
H₂(g) → 2H⁺(aq) + 2e⁻
Reduction:
O₂(g) + 4H⁺(aq) + 4e⁻ → 2H₂O(l)
Overall reaction:
2H₂ + O₂ → 2H₂O
Write the conventional cell representation for a hydrogen fuel cell in acidic conditions.
Pt(s)│H₂(g)│H⁺(aq) || O₂(g)│H⁺(aq) , H₂O(l)│Pt(s)
Write the conventional cell representation for a hydrogen fuel cell in alkaline conditions.
Pt(s)│H₂(g)│OH⁻(aq) , H₂O(l) || O₂(g)│H₂O(l) , OH⁻(aq)│Pt(s)
Write the half equations for a hydrogen fuel cell in alkaline conditions, and so write an overall reaction.
Oxidation:
H₂(g) + 2OH⁻(aq) → 2H₂O(l) + 2e⁻
Reduction:
O₂(g) + 2H₂O(l) + 4e⁻ → 4OH⁻(aq)
Overall reaction:
2H₂ + O₂ → 2H₂O
Draw a hydrogen fuel cell (in alkaline conditions).
The central electrolyte allows ions and molecules to move through it but not electrons.
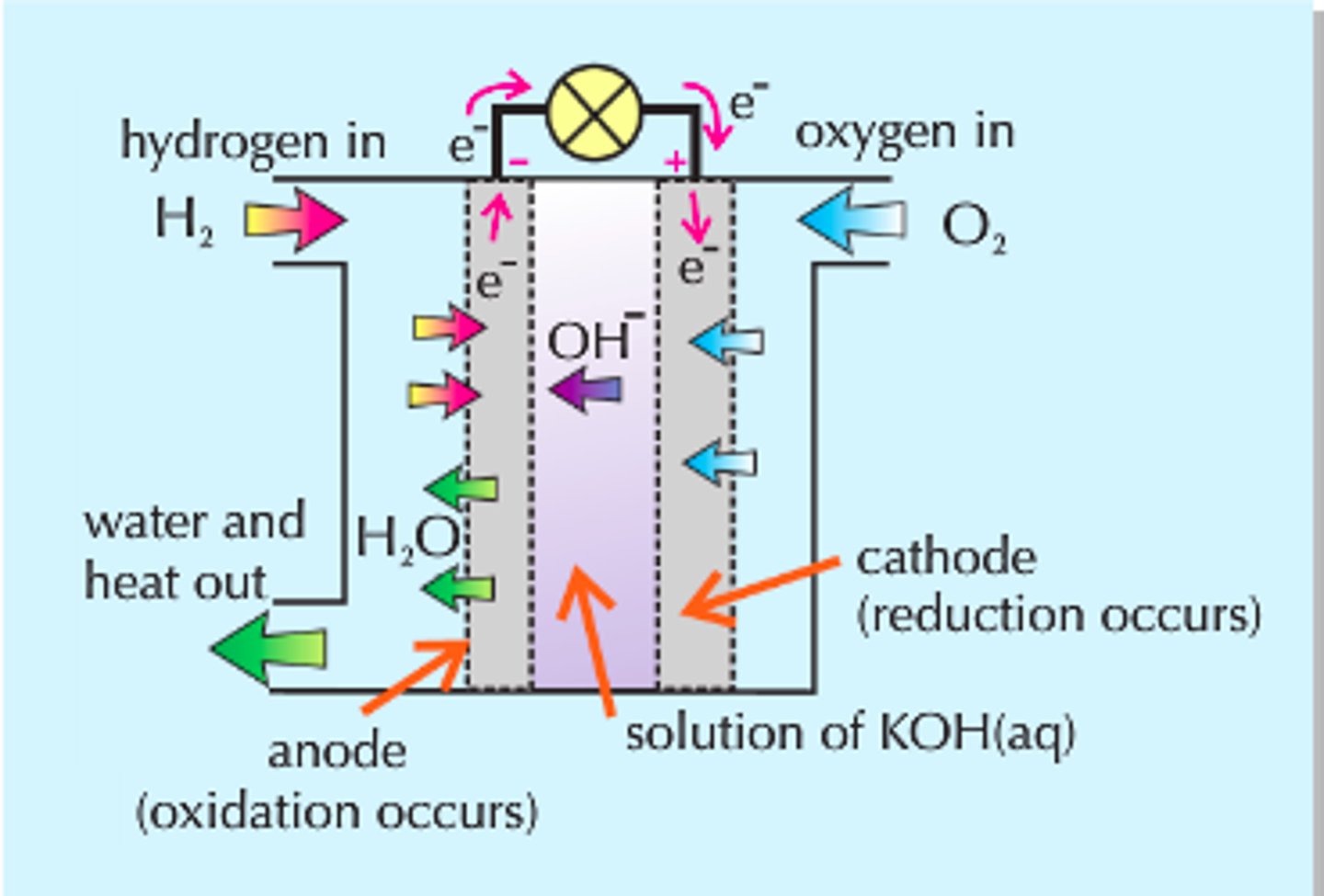
Suggest why fuel cells may not be carbon neutral.
An energy source may have been used to obtain the fuel that is not carbon neutral (such as splitting of hydrocarbons to produce hydrogen).
Write the half equations for an ethanol fuel cell, and so write an overall reaction.
Oxidation:
C₂H₅OH(l) + 3H₂O(l) → 2CO₂(g) + 12H⁺(aq) + 12e⁻
Reduction:
12H⁺(aq) + 3O₂(g) + 12e⁻ → 6H₂O(l)
Overall:
C₂H₅OH(l) + 3O₂(g) → 2CO₂(g) + 3H₂O(l)
Which electrode is positive?
Cathode
Which electrode is negative?
Anode