Chemistry - Ch 13: Solids and Modern Materials
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
crystalline
(a solid) composed of atoms or molecules arranged in structures with long-range order
e.g. snowflakes, crystals with smooth faces and well-defined angles
amorphous
(a solid) composed of atoms or molecules with no long-range order
e.g. glass, chocolate
x-ray diffraction
a laboratory technique that allows for determining the arrangement of atoms in a crystal for measuring the distance between them
crystalline lattice
the regular arrangement of atoms within a crystalline solid
represented with unit cell
unit cell
a small collection of atoms, ions, or molecules in a lattice
the smallest divisible unit of a crystal that, when repeated in three dimensions, reproduces the entire crystal lattice
e.g. cubic unit cell
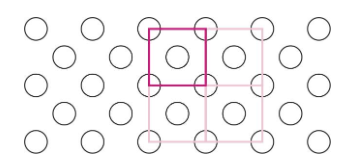
3 types of cubic unit cells
simple cubic (SC)
body-centered cubic (BCC)
face-centered cubic (FCC)

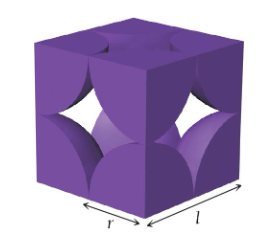
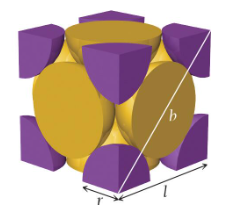
simple cubic unit cell
a cube with one atom at each corner
edge length: l = 2r
net atoms per cell: 1/8 × 8 = 1
coordination number: 6
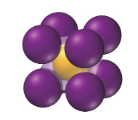
coordination number
the number of atoms with which each atom in a unit cell is in direct contact
the number of atoms with which a particular atom can strongly react
body-centered cubic unit cell
a cube with one atom at each corner and one atom in the center of the cube
atoms do not touch along each edge of the cube, but touch along the diagonal line going from corner to corner of the cube
edge length: l = 4r/√3
net atoms per cell: (1/8 × 8) + 1 = 2
coordination number: 8
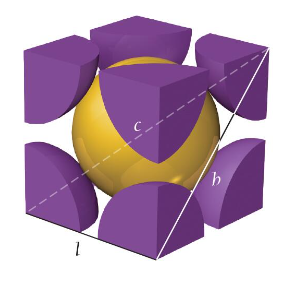
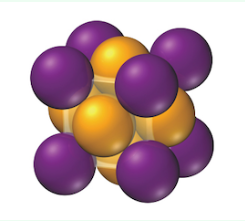
face-centered cubic unit cell
a cube with one atom at each corner and one atom in the center of each cube face
atoms do not touch along each edge of the cube; touch along the face diagonal (like BCC)
edge length: l = 2r√2
net atoms per cell: (1/8 × 8) + (1/2 × 6) = 4
coordination number: 12
3 categories of crystalline solids
molecular, ionic, atomic
classified based on the individual particles that compose the solid
3 categories of atomic: nonbonded, metallic, network covalent
molecular solids
solids in which the composite particles are molecules
e.g. ice (solid H2O), dry ice (solid CO2)
held together by IMFs
tend to have low to moderately low melting points
specific properties depend on the types of IMFs between molecules and structure of molecule and crystal structure of the solid
some can be polymorphs
polymorphs
the different crystal structures that can sometimes exist for the same compound
different polymorphs can have different properties, e.g. melting point and solubility
e.g. ritonavir used for HIV and AIDS, chocolate
ionic solids
solids in which the composite particles are ions
e.g. NaCl, CaF2
forces holding them together are strong coulombic forces (ionic bonds); stronger than IMFs
tend to have much higher melting points than molecular solids, because ionic bonds are stronger than IMFs
atomic solids
solids in which the composite particles are individual atoms
e.g. solid Xe, Fe, SiO2
3 categories: nonbonding, metallic, network covalent (all held together by a different kind of force)
nonbonding atomic solids
atomic solids held together by relatively weak dispersion forces
in order for interactions to be maximized, closest-packed structures are formed (maximize coordination numbers, minimize distance between composite units)
very low melting points that increase uniformly with molar mass
noble gases in solid form are the only example of this solid
metallic atomic solids
atomic solids held together by metallic bonds (represented by interaction of metal cations with the surrounding electron sea)
not directional; many metals tend to form closest-packed crystal structures
have varying strengths and varying melting points
network covalent atomic solids
atomic solids held together by covalent bonds
e.g. diamond, graphite, silicon dioxide
crystal structures are more restricted by the geometrical constraints of covalent bonds (tend to be more directional than IMFs, ionic bonds, or metallic bonds)
do not tend to form closest-packed structures
very high melting points because of strength of covalent bonds
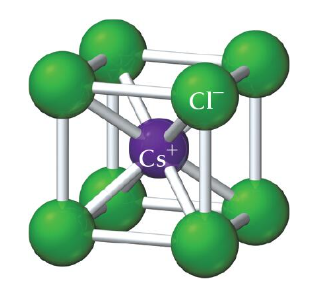
cesium chloride (CsCl) structure
example of ionic compound with cations and anions of similar size
Cl- ions occupy lattice sites of a simple cubic (corners), 1 Cs ion lies in the center of the cell
coordination number is 8 (each Cs ion is in direct contact with 8 Cl ions and vice versa)
Cs:Cl ratio of 1:1
CaS has same structure
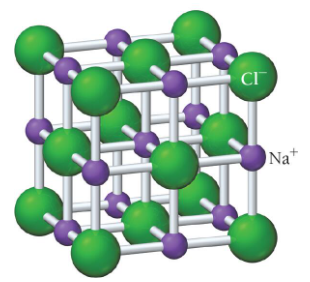
sodium chloride (NaCl) structure
example of ionic compound with different ion sizes
Cl- has a much larger radios than Na+
crystal structure must accommodate disproportionate sizes
coordination number is limited by the number of Cl anions that can fit around the smaller Na cation
coordination number: 6
rock salt structure: Cl- anions occupy lattice sites of a face-centered cubic structure, Na+ cations occupying holes between anions
each unit cell contains 4 Cl- ions and 4 Na+ ions
ratio of 1:1
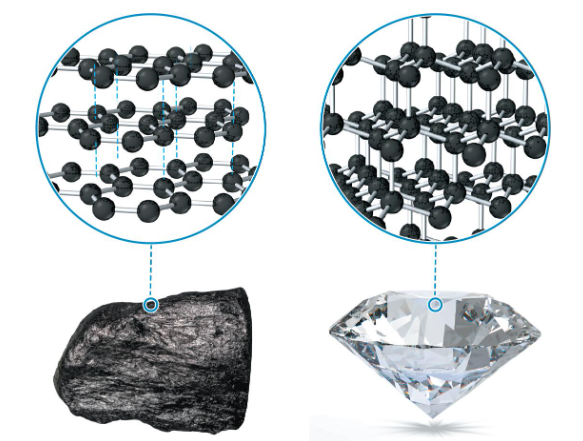
2 crystalline forms of carbon
graphite: flat sheets of C atoms covalently bonded together as interconnected hexagonal rings
no covalent bonds between sheets, only LDFs, so sheets slide past each other with relative ease
diamond: face-centered cubic structure with an additional C atom in half of the holes beneath each corner atom
each C atom is covalently bonded to 4 others at the corners; structure extends throughout entire crystal
very high melting point, does not conduct electricity, very hard
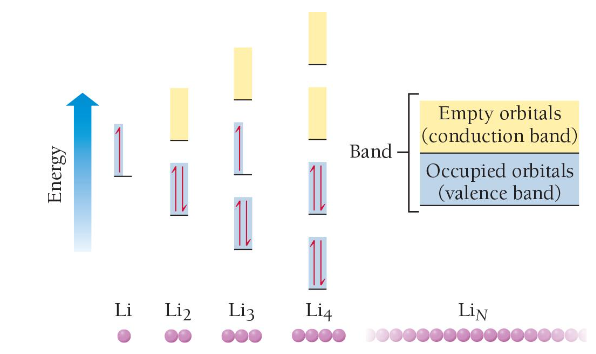
band theory
a model for bonding in atomic solids based on molecular orbital theory in which atomic orbitals combine and become delocalized over the entire crystal
applies to metallic and covalent solids
electrons in a molecule occupy the lower-energy bonding orbital, leave antibonding orbital empty
eventually, there are so many molecular orbitals, energy spacings in between become very small and form a band of energy levels (no longer discrete)
valence band: one half of orbitals in the band (N/2) are bonding molecular orbitals, contain the N valence electrons (at 0 K)
conduction band: other half of orbitals are antibonding and completely empty (at 0 K)
above 0 K, electrons can make transition from valence to conduction band
band gap: energy gap in semiconductors and insulators that exists between valence and conduction bands
no band gap in conductors
band gap decreases as you move down a column of elements because increasing radius reduces overlap between orbitals on neighboring atoms → reduces energy differences between conduction and valence bands
doping
adding trace amounts of a dopant to a substance to modify its properties
can result in n-type or p-type semiconductors
n-type semiconductor
semiconductor prepared by doping with a valence-electron-rich element to act as charge carriers in the conduction band
results in a more negative semiconductor
e.g. doping Si with P (dopant is further right on the periodic table than the original element)
p-type semiconductor
semiconductor prepared by doping with a valence-electron-deficient element to act as charge carriers in the conduction band
results in a more positive semiconductor
e.g. doping Si with Ga (dopant is further left on the periodic table than the original element)
Ga atoms trap some of the electrons in Si’s valence band; results in electron “holes” (empty molecular orbitals) in valence band, allows for movement of electrical current