Photogrammetry Exam 1
1/125
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
126 Terms
Wavelenght
distance between 2 peaks or 2 troughs
inversely proportional to frequency
inversely proportional to energy
lambda
units are micrometers
frequnecy
inversely proportional to wavelength
proportional to energy
atmospheric walls
opaque to solar or other electromagnetic radiation
white spaces between atmospheric transmissions
obstructs transmission
wavelength ranges
ultraviolet
blue
green
red
near infrared
photographic infrared
mid-infrared
thermal infrared
microwave
visible light
0.4 μm to 0.7 μm
blue
green
red
ultraviolet
less than 0.4 μm
blue
0.4 - 0.5 μm
band 1
green
0.5 - 0.6 μm
band 2
red
0.6 - 0.7 μm
band 3
near infrared
0.7 - 1.3 μm
band 4
photographic infrared
0.7 - 0.9 μm
mid infrared
1.3 - 3 μm
thermal infrared
greater than 3 μm
microwave
1 mm - 1 m
emitted
energy coming from inherent heat or energy absorbed
energy coming off something
thermal
metabolic processes
absorbed radiation
reflected
light that bounces off something and then comes back
speed of light equation
c=νλ
speed of light
constant
slower in water or air than in a vacuum
3×10^8 m/s in a vacuum
electromagnetic radiation
electromagnetic energy composed of discrete units called protons or quanta
energy of quanta
Q=hc/λ
joules pf energy = (Planck’s constant x speed of light)/ wavelength
quantum energy
inversely proportional to wavelength
E=hc/λ
flux
how many particles an area is getting
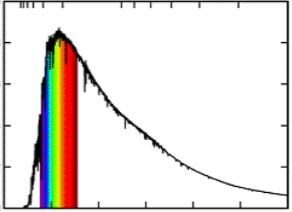
photon flux (Φ)
number of photons per second per unit area
doesn’t incorporate energy or wavelength
number of particles/ m² x s
power density (H)
incorporates energy and wavelength
photon flux times energy
Φ x quantum energy (hc/λ)
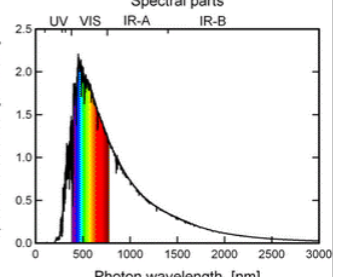
spectral irradiance
power density / Δλ
energy from the sun
there is a rapid decrease in spectral irradiance as the wavelength increases
there is a more gradual decrease in photon flux as the wavelength increases
yellow has the most spectral irradiance and highest proton flux
waves and particles
distinct but related
energy per quantum decreases as wavelength increases
when wave-packets are spatially localized, they are considered particle like
energy per unit basis
based on how many photons and the amount of energy per photon
sunlight
electromagnetic radiation
visible light is a small subset of the electromagnetic spectrum
light
wave with particular wavelength
packets of particles of energy, photons
both wave-like and particle-like
either absorbed, reflected, or transmitted
quanta
total energy of light is made up of indistinguishable energy elements
blue light
has higher energy but shorter wavelength
red light
has lower energy but longer wavelength
the photon flux of a higher energy photon to give a specific power density
will be lower than the photon flux of a lower energy photon required to give the same power density
spectral irradiance (F)
gives the power density at a particular wavelength
F = Φ x E x (1/Δλ)
short wavelength
high energy
high frequency
long wavelength
low energy
low frequency
photon flux units
photons per square meter per second
quantum energy units
joules per photon
spectral irradiance units
watts per square meter per micrometer
power density units
watts per square meter
spectral irradiance graph

photon flux graph

2 parts of optical wavelengths
-wavelength band
-spectral band
defined regions
1 micrometer
1000 nanometers
atmospheric windows
atmosphere is largely transparent
put sensors here
spectral bands through time
are very similar
landsat data continuity act
short wave infrared 1
band 5
short wave infrared 2
better to separate vegetation from soil
standard false color composite
NIR → Red
Red → Green
Green → Blue
432
true color composite
actual colors we would see
pixel
picture element
ground resolution cell
spatial resolution
horizontal dimension
side of a ground resolution cell
identify feature
shows you the 6 wavelength bands the satellite records
spectral signiture
relates to color/hue
reflections as a function of wavelength
material type and condition
Incident energy
=reflected energy + absorbed energy +transmitted energy
variation of wavelenght
gives us color
how we see in response to reflected light
1
= reflectance + absorptance + transmittance
unitless
scattering
when you hit something and it moves off in all directions
unpredictable diffusion of radiation by particles in the atmosphere
how we see light in our shadow
every bit of light we see has some of this, have to correct for remote sensing
doesn’t change wavelength
absorption
sucks energy in
effective loss of energy
taking in of energy at particular wavelengths by atmospheric constitutes
causes object to heat up
Rayleigh Scattering
Particle diameter is larger than wavelength
why sky is blue and sunsets are red
cause of haze in imagery
caused by aerosols (small molecules)
wavelength specific
shorter wavelength have more scattering
Nonselective Scattering
Particle diameter is smaller than wavelength
all wavelengths are effected equally (not wavelength specific)
water droplets cause this
why clouds are white
Mie Scattering
particle diameter equals wavelength
dominates in slightly overcast or hazy conditions
water vapor and dust cause this
diffuse reflectance
scatters in the atmosphere and interferes with reading
path radiance
Top atmospheric gases that are absorbers
Water (H2O)
Carbon Dioxide (CO2)
Methane (CH4)
Carbon Dioxide units in atmosphere
Parts per million
Methane units in atmopshere
parts per billion
H2O units in atmosphere
Percent
Atmospheric water vapor feedback
Positive: clouds
Negative: humidity
When something gets hotter
more energy is emitted
the energy emitted out is a shorter wavelength
Ozone
short wavelengths are absorbed
long wavelengths are released out
keeps UV light out of atmosphere
chlorophyll
absorbs red and blue light
leaves green
sources of emitted energy
human endothermic system
solar fusion
thermal energy
blackbody
no light is coming out
unreflective and absorbs across the spectrum
as it heats up it emits more energy
emitted energy falls along Planck’s curve
emissivity = 1
hot fires
have a wavelength peak of 3 micrometers
can see with sensors
thermal scanners see 3000 - 15000 nm
spectral resolution
how finely we divide the electromagnetic spectrum
color picture has 3 bands
where you are in respect to where sun emits, smaller wavelengths more ideal
as you get longer wavelengths, you get less solar radiation
more bands: less energy
Panchromatic band
giant band 8
the more you divide up spectral resolution
higher resolution
you lose energy
spatial resolution becomes more coarse
finer spatial resolution
decreased energy
how to get more photons to increase signal/noise ratio
use an active sensor
coarsen spectral resolution
active sensor
sends energy out to illuminate objects
flash photography
passive sensor
relies on just light emitted or reflected from the environment
graybody
not completely black but still has a Planck Curve
emissivity less than one
same peak and shape of blackbody
doesn’t vary by wavelength
selective radiator
if emissivity varies with wavelength
geometric manner of reflectance
if something is smooth, you will see hotspots
specular reflector
most smooth
one angle of incidence and one angle of reflectance
mirror
Lambertian surface
diffuse reflector
most rough
one angle of incidence and many equal angles of reflection
rough surfaces
are better for remote sensing
with respect to wavelength of energy
leaves
have lots of infrared reflecting off it
most visible wavelengths are absorbed in leaves
overall reflectance is low
higher green reflectance than red or blue
needles less reflecting (NIR)
variability in spectral signals
atmospheric effects
phenology or other temporal changes (snow…)
differential illumination (shadows)
feature conditions (drought…)
mixed pixels/ spectral mixture
mixtures of soil, veg, or water in a pixel
can lighten or darken spectral signature
dirt added to a pond would increase the pond’s NIR reflectance
radiometric resolution
ability of remote senses system to record many levels of a value
more bins = more resolution
100% vs A-F vs Pass Fail
Landsat 1-3
6 bit
Landsat 4-7
8 bit
Landsat 8-9
12 bit
Base 10
(_ x 10^0) + (_ x 10^1) + (_ x 10²) …
Base 2
(_ x 2^0) + (_ x 2^1) + (_ x 2²) …
feature space
n-dimensional space with all possible combinations of DN’s/e’s ???
Classification schemes
informational classes
use an existing one
principles of classification schemes
hierarchical
exhaustive
mutually exclusive