C2 - Atoms, molecules, etc. (Gen. Chem 1)
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1
atomic mass unit (amu)
1 amu = 1.66 × 10-24 g (1/12 of a C-12 atom)
= g/mol
2
isotope notation
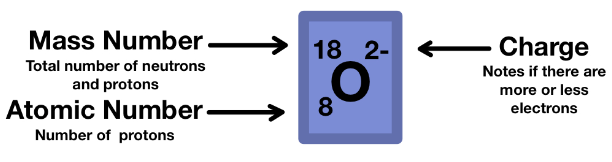
3
atomic weight
avg. atomic weight, based on isotope abundance
4
cation nomenclature
[name] + ion
X[charge], X(charge)
[prefix] - ium + ion
5
anion nomenclature
-ide + ion
oxyanions
-ate (common)
-ite (1 less O)
per- (1 more O than -ate)
hypo- (1 less O than -ite)
hydrogen + [name] + ion
6
ionic compound nomenclature
[cation] + [anion]
7
molecular compound nomenclature
[leftmost, bottommost] + [next] -ide
# prefix
8
acid nomenclature
-ate to -ic
-ite to -ous
-ide → -ic (hydro-)
retain prefixes