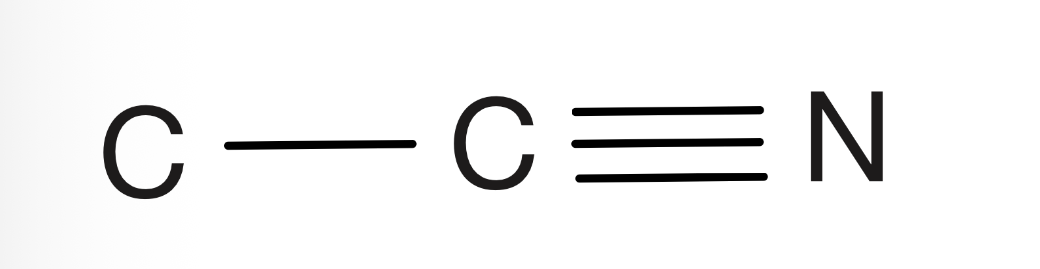Organic functional groups
1/18
Earn XP
Description and Tags
Week 1
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
Alkanes
All carbon bonds are single
Alkenes
At least one double carbon bond
Alkynes
At least one triple carbon bond
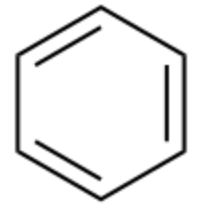
Arenes
Benzene rings (EX: C6H6)
Halides
Carbon connected to F, Cl, Br, or I
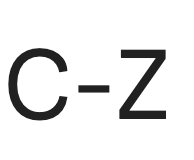
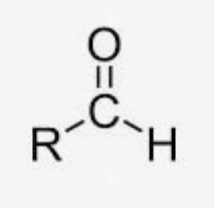
Aldehydes
Carbon with an oxygen double bond, and connected to either two hydrogens or a hydrogen and a carbon
Ketone
Carbon with an oxygen double bond, and is connected to two carbons
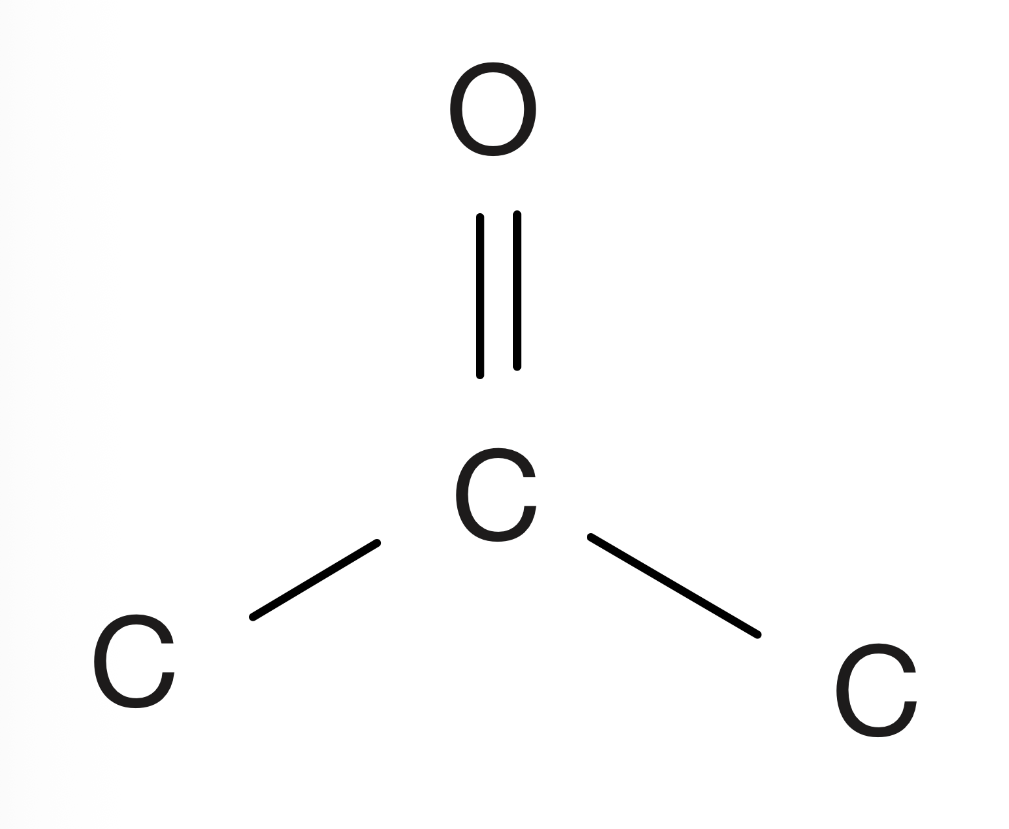
Amine
Nitrogen connected to three carbons, three hydrogens, or a mixture of the two, no carbon double bonds are allowed
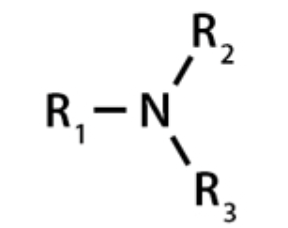
Alcohol
Carbon with an sp3 orbital connected to an OH
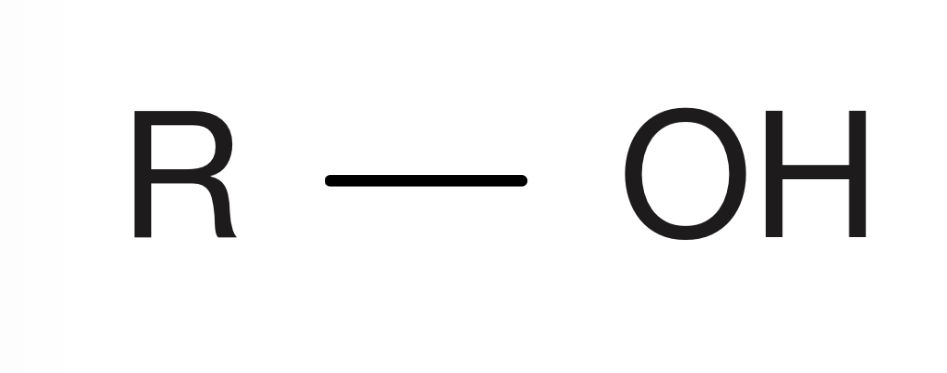
Carboxylic acid
Carbon with an oxygen double bond, and connected to an OH and a hydrogen or a carbon
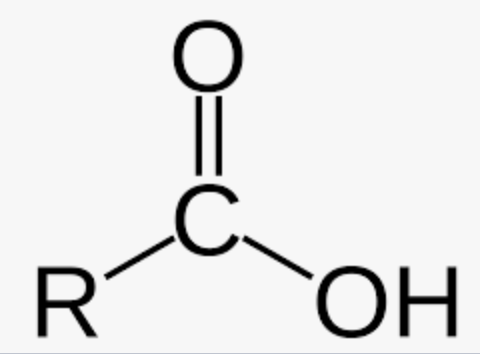
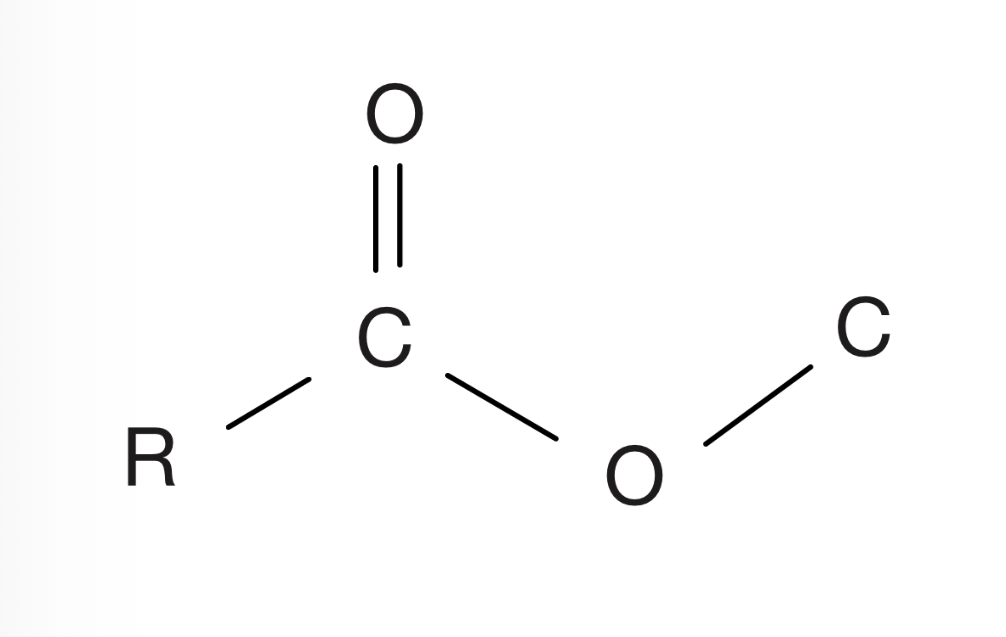
Ester
Carbon with an oxygen double bond and connected to a carbon or hydrogen, and connected to an oxygen which is connected to another carbon that is never in a double bond
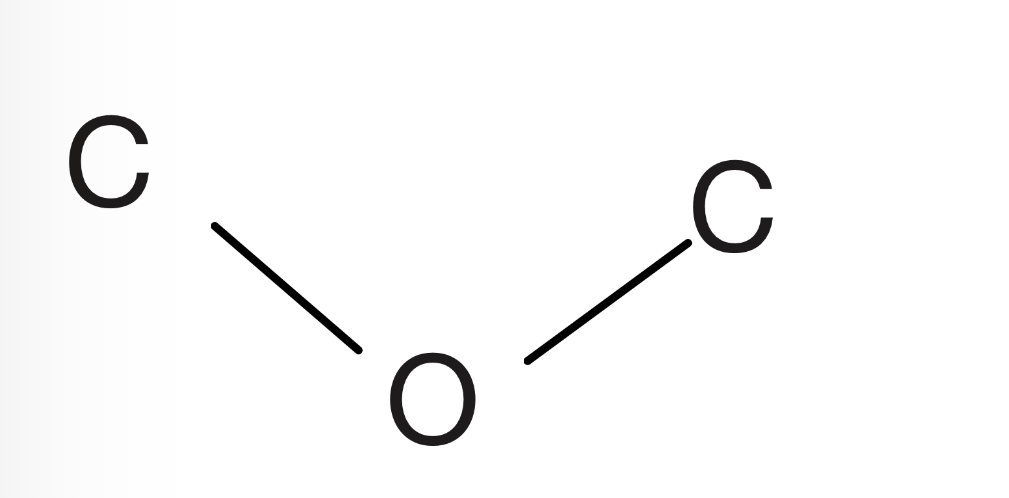
Ether
Oxygen connected to two carbons of which have to be sp3
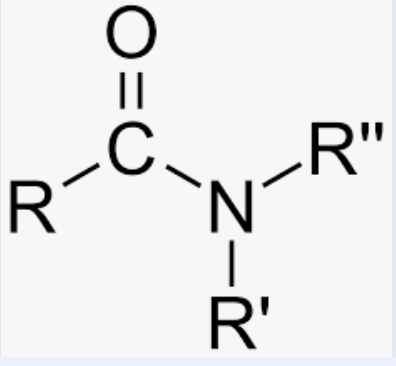
Amide
Carbon with an oxygen double bond, and connected to a carbond or hydrogen, and connected to a nitrogen which is connected to either two hydrogens, two oxygens, or one of each
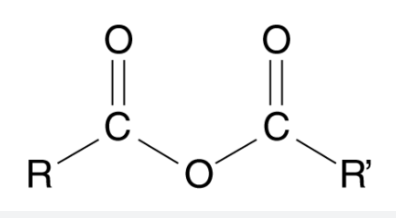
Anhydride
Oxygen connected to two carbons each with oxygen double bonds, and each connected to their own carbon or hydrogen
Acid halide
Carbon with an oxygen double bond, and connected to hydrogen or carbon, and connected to either F, Cl, Br, or I
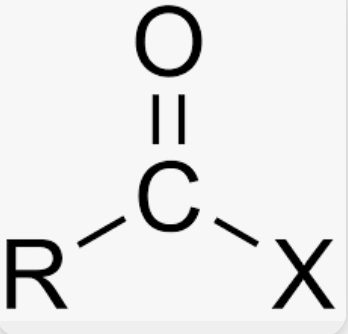
Thiol
Carbon connected to SH
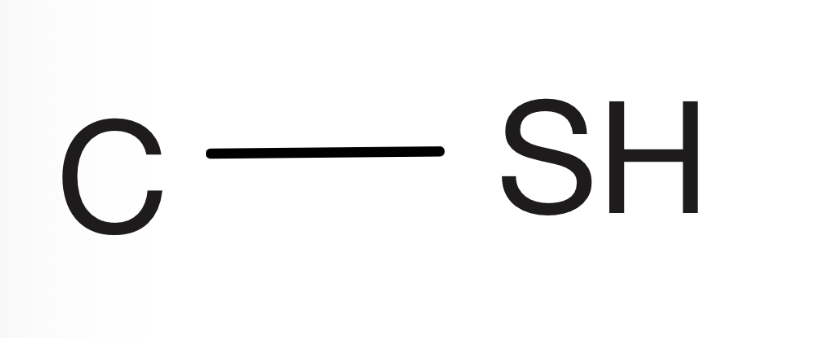
Disulfide
Two sulfurs bonded in the center, with two carbons on either side, the carbons cannot have a double bond to oxygens
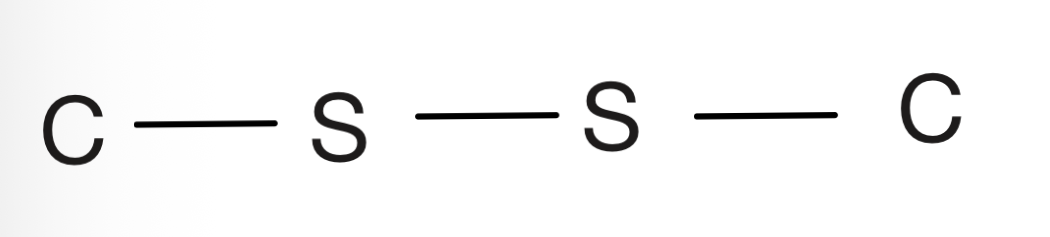
Nitro
Carbon bonded to NO2
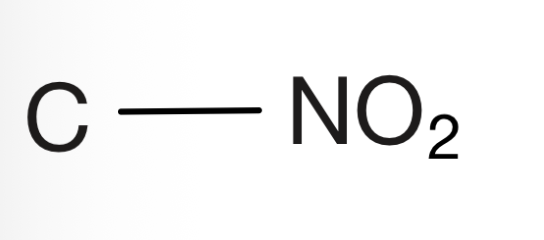
Cyano
Carbon bonded to carbon and triple bonded to nitrogen a