A2 Physics Nuclear Physics
1/17
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
formula for equivalence between energy and mass
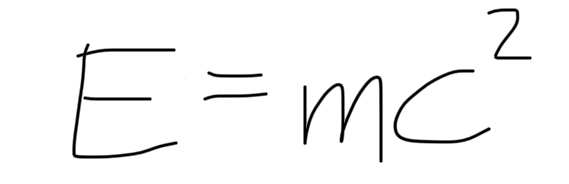
what is mass defect
difference between mass of nucleus and sum of individual protons and neutrons
what is binding energy
energy required to separate the nucleons to infinity
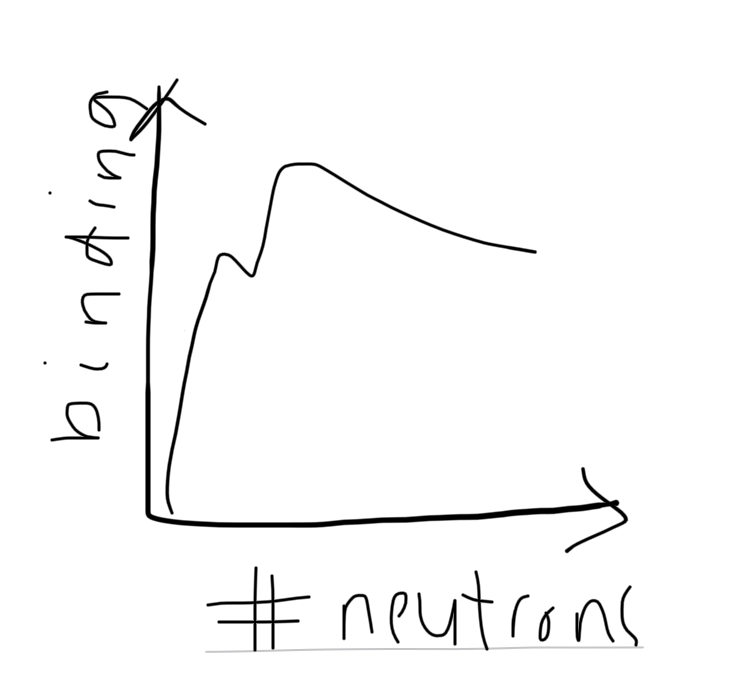
sketch the variation between binding energy per nucleon with nucleon number
what is nuclear fusion
fusing of two small nuclei to produce larger nucleus
what is nuclear fission
splitting of large nucleus to smaller nuclei
what is the relevance of binding energy per nucleon to nuclear reactions like nuclear fusion and fission
at low values of A (nucleon number), so lower binding energy per nucleon, nuclei go under fusion
at higher values of A (nucleon number), meaning higher binding energy per nucleon, nucleus undergoes fission
formula for energy released in nuclear reactions
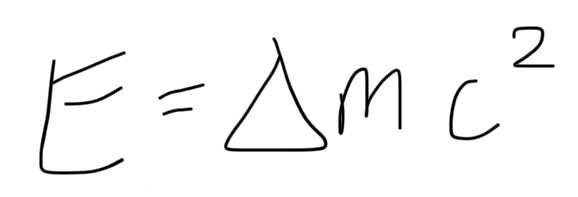
what are the conditions of radioactive decay
it is both random and spontaneous
what does random mean
exact time of decay of a nucleus cannot be predicted
what does spontaneous mean
cannot be influenced by external environmental factors
what is activity
number of decays per unit time
unit of activity
Bq
what is decay constant (lambda)
probability that an individual nucleus will decay per unit time
formula for activity
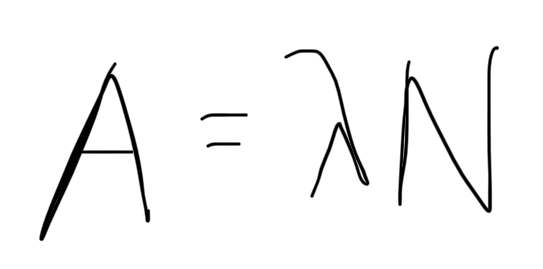
what is half-life
time taken for activity to halve
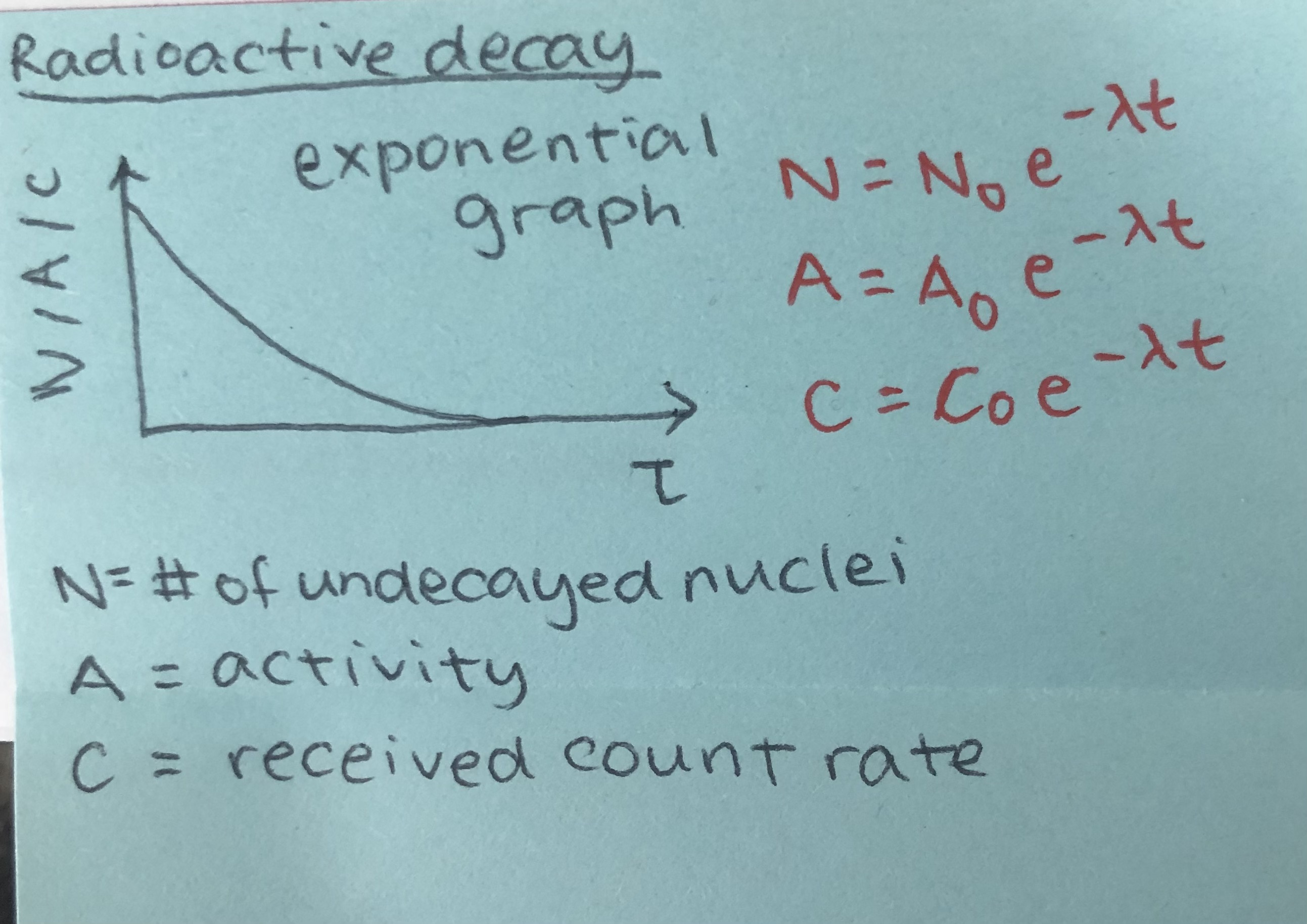
sketch the graph of radioactive decay and state the 3 formulas for radioactive decay
how can the random nature of radioactive decay be proved
using fluctuations in count rate