Khan Academy: Biochem Review
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
What is the difference between L and D amino acids?
How are Peptide bonds formed, how are they stabilized?
All proteogenic amino acids in the human body are L, except glycine (achiral).
Cysteine is still L, but it has an (R) absolute configuration due to sulfur group in its side-chain
Peptide bonds form through a condensation (dehydration) reaction via nucleophilic acyl substitution, resulting in an amide bond between amino acids.
The nucleophilic attack comes from the amino group (-NH₂) of one amino acid attacking the carbonyl carbon (C=O) of the carboxyl group (-COOH) of another.
This leads to the formation of an amide bond (peptide bond) with the loss of a water molecule (H₂O).
This is a nucleophilic acyl substitution reaction, not simple nucleophilic addition.
The peptide bond has resonance between the carbonyl oxygen and amide nitrogen, giving it partial double-bond character, rigidity, and stability.
The polypeptide backbone follows a repeating N–Cα–C' pattern, providing structural consistency while the R-groups (side chains) vary and define the properties of the protein.
Polypeptides have directionality, always synthesized and read from N-terminal → C-terminal, with the N-terminal having a free amine and the C-terminal having a free carboxyl group.
2 Ways of Amino Acid Cleavage
Polypeptides can be cleaved nonspecifically with acid and heat
Polypeptides can be cleaved specifically using proteases, which recognize particular amino acids.
TRYPSIN: Cleaves at C-terminal of arginine (R) and lysine (K)
Trypsin is secreted by the pancreas, specifically from the exocrine cells of the pancreas in the form of its inactive precursor, trypsinogen. It is then activated in the small intestine by enteropeptidase to become trypsin, which helps break down proteins.
CHEMOTRYPSIN: Cleaves at the C-terminal of aromatic residues (F, W, Y)
Chymotrypsin is also secreted by the pancreas in an inactive form called chymotrypsinogen. It gets activated in the small intestine by trypsin, which converts it into its active form, chymotrypsin, to aid in protein digestion.
At physiological pH molecules are typically COO- and NH3+ (neutral)
At physiological pH (~7.4), Aspartate and Glutamate exist in their deprotonated (-COO⁻) forms, making them NEGATIVELY charged and important for protein structure and function.
Will be COO- and COO- and NH3+
Overall -1
pKa (acid dissociation constant) v. pI (isoelectric point)
pKa refers to the pH at which HALF of a specific ionizable group (e.g., carboxyl or amino group) is protonated and HALF is deprotonated.
pI (isoelectric point) is the pH at which the entire molecule (amino acid or protein) has no net charge—meaning its positive and negative charges balance out.
pH<pKa means the group is protonated (COOH instead of COO- or NH3+ instead of NH2)
pH>pKa means the group is deprotonated (COO- or NH2)
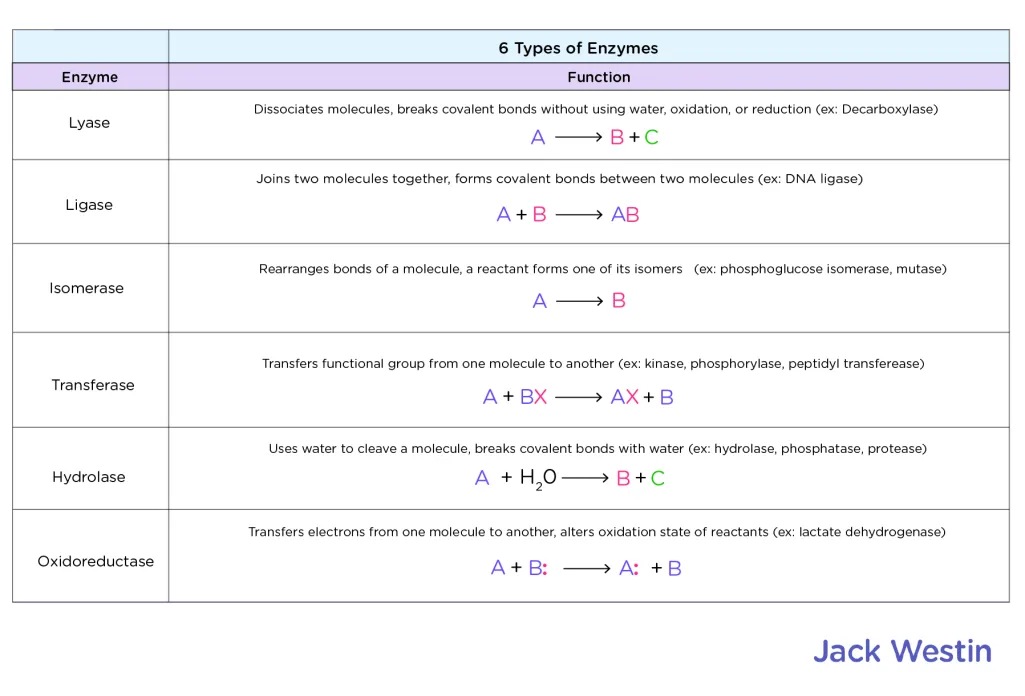
What are the six types of enzymes to know?
Which lipids are amphipathic and which ones are not?
Phospholipids
Main components of cell membranes, are amphipathic as hydrophilic head (usually phosphate) group and hydrophobic fatty acid tails
Cholesterol
Found in cell membrane, precursor for steroid hormones, maintain fluidity
Has hydrophilic hydroxyl (OH) group and hydrophobic steroid ring structure
Fatty Acids
Carboxylic acids with long hydrocarbon chains
Carboxylic acid group is hydrophilic, CH chians are hydrophobic
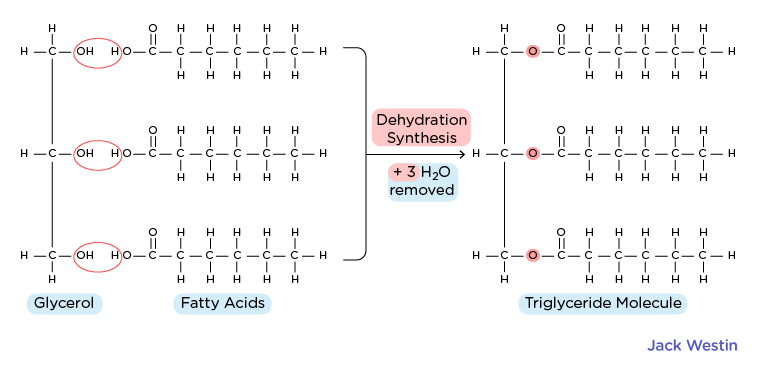
Triacylglycerols/Triglycerides (IMAGE 2)
Composed of glycerol molecule esterified to three fatty acid chains
Thus is ENTIRELY hydrophobic and lacks a hydrophilic region
NOT AMPHIPATHIC
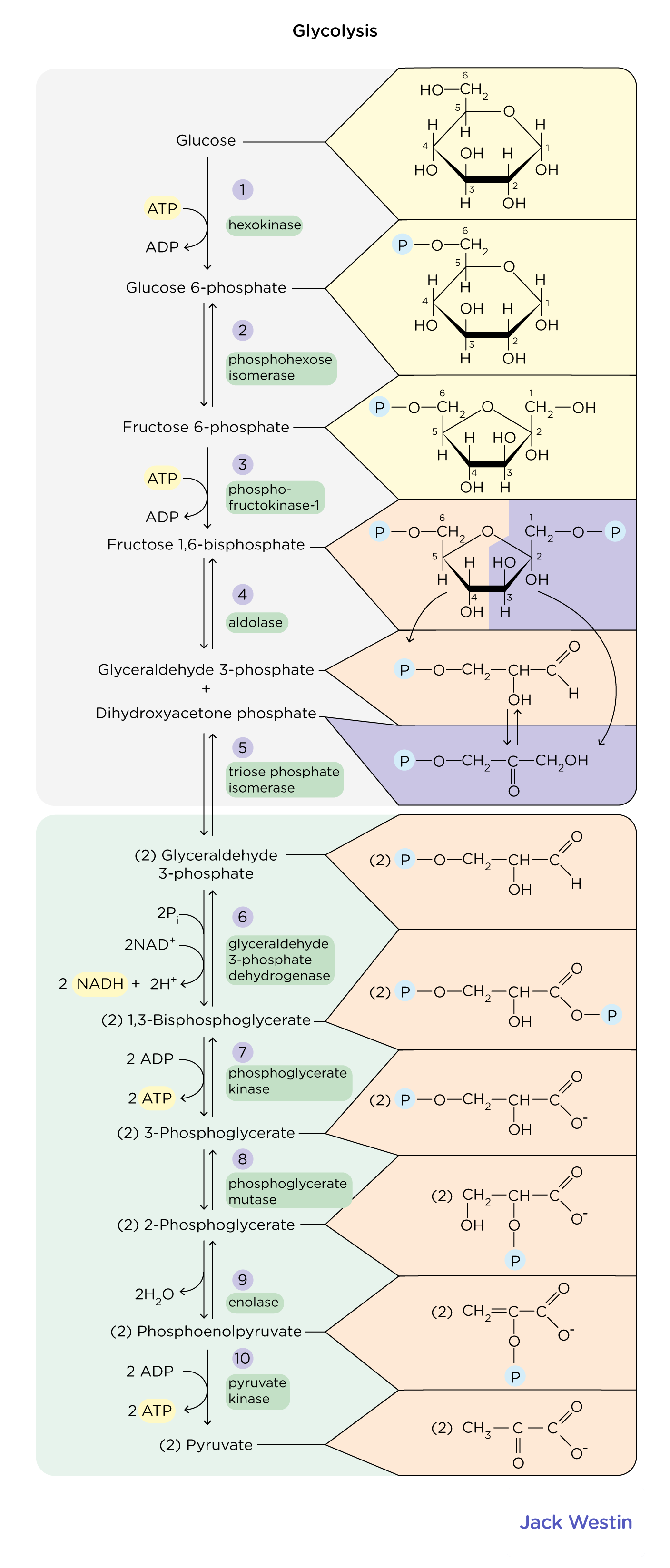
What does pyruvate kinase do in glycolysis?
Pyruvate kinase breaks down phosphoenolpyruvate (PEP) down robbing it of a phosphate and giving it to ATP to make Pyruvate + AATP
This is an exergonic reaction coupled to endergonic reaction to drive ATP synthesis
Which amino acids yield or do not yield intermediates that enter the TCA cycle?
TCA Cycle
Oxidizes acetyl-CoA to produce various intermediates and make ATP
Glucogenic Amino Acids
Broken down into intermediates that can be converted into glucose via gluconeogenesis
Alanine, Arginine, Asparagine, Aspartate, Cysteine, Glutamic Acid, Glutamine, Glycine, Histidine, Methionine, Proline, Threonine, Serine, Valine
Ketogenic Amino Acids
Broken down into intermediates that can be converted into ketone bodies or other molecules that can be used for ATP production other than glucose
Ketone bodies are produced in liver during periods of low glucose availability
Leucine and Lysine
Remember KILL is Ketogenic
Ketogenic: Leucine and Lysine
Rest are glucogenic
Both Gluco and Ketogenic Amino Acids
PITT is on both sides
Phenylalanine, Isoleucine, Tryptophan, Tyrosine
Gluconeogensis
Process by which body generates glucose from non-carb sources when glucose is scarce like during fasting or low-carb states
Tyrosine
Catabolized to form fumarate
Arginine
Catabolized to form ornithine which is converted into alpha-ketoglutarate an intermediate in TCA
Isoleucine
Catabolized to form acetyl-CoA and propionyl CoA, which can enter the citric acid cycle as acetyl-CoA or be converted to succinyl-CoA anothre intermediate in the cycle
Ketogenic and glucogenic amino acids enter the TCA cycle at different points after being metabolized into intermediates:
Glucogenic Amino Acids
Converted into pyruvate → Enters the cycle via oxaloacetate
Example: Alanine, Serine, Cysteine
Converted into TCA intermediates:
α-Ketoglutarate (via glutamate) → Glutamine, Proline, Arginine, Histidine
Succinyl-CoA → Methionine, Isoleucine, Valine, Threonine
Fumarate → Phenylalanine, Tyrosine
Oxaloacetate → Aspartate, Asparagine
Ketogenic Amino Acids
Converted into Acetyl-CoA or Acetoacetate, which enter the TCA cycle via citrate synthase
Example: Leucine, Lysine (exclusively ketogenic)
Both ketogenic & glucogenic: Isoleucine, Phenylalanine, Tyrosine, Tryptophan
Key point: Acetyl-CoA cannot be used for gluconeogenesis, while TCA intermediates from glucogenic amino acids can be.
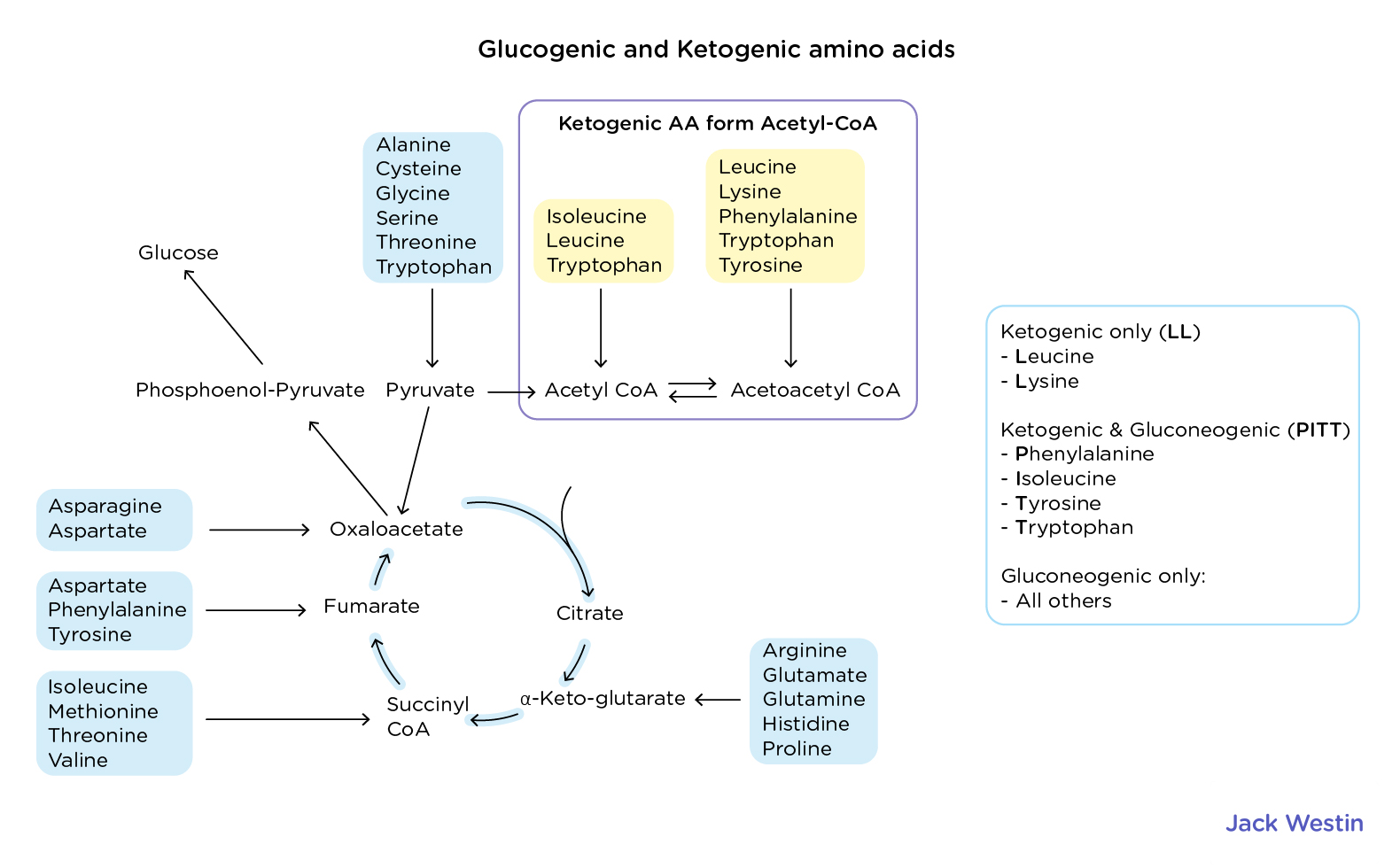
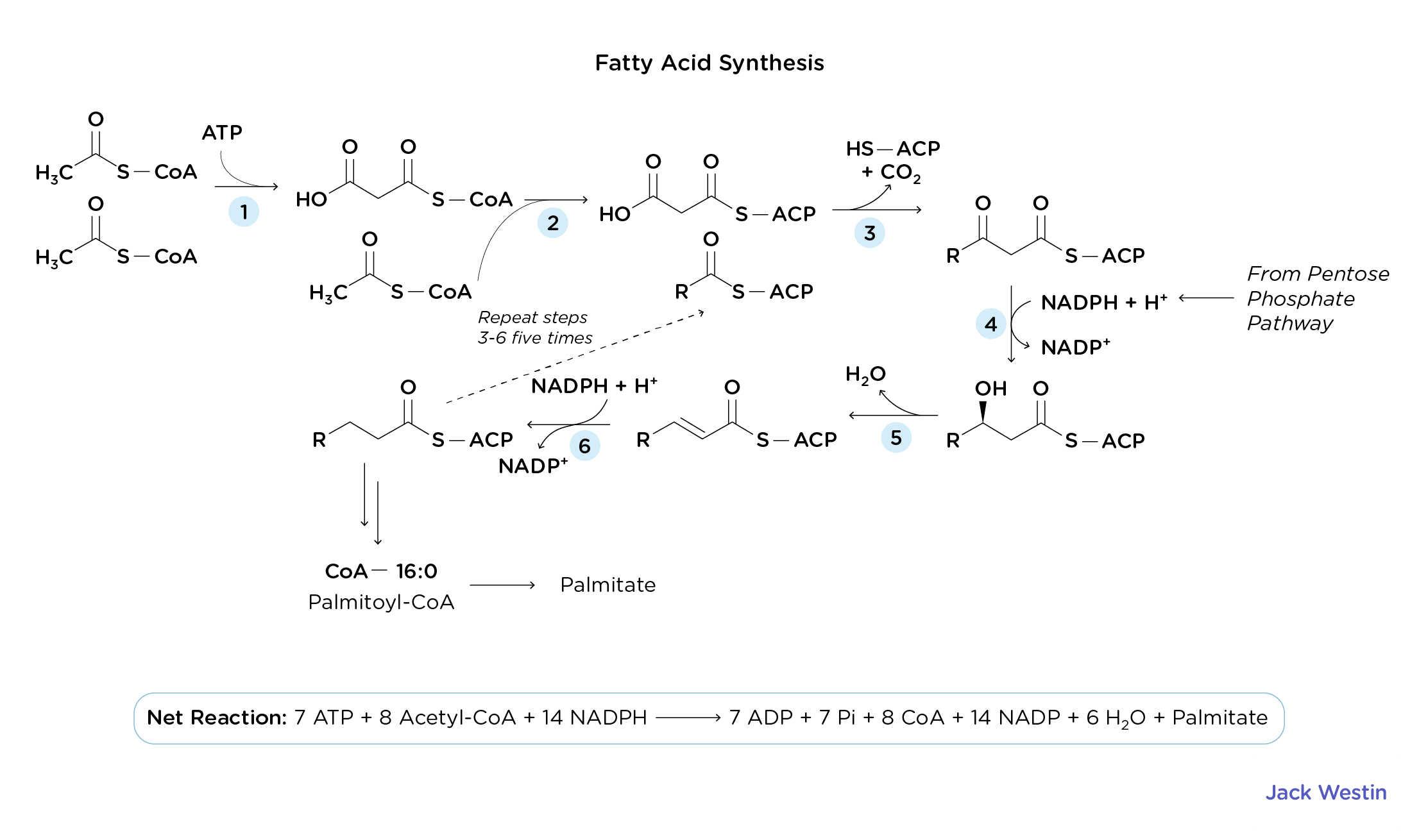
Describe fatty acid synthesis
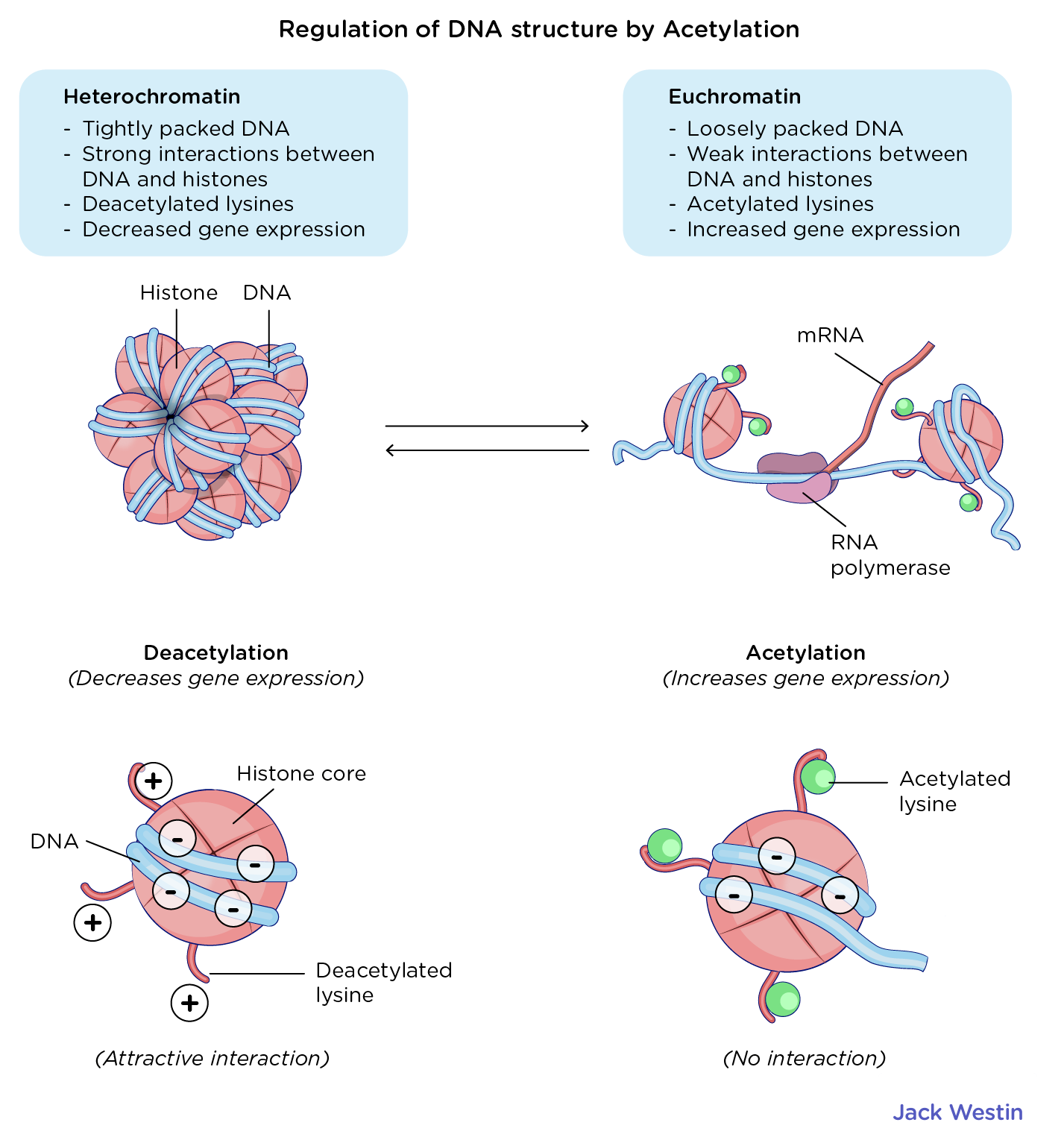
How does Acetylation Regulate DNA Structure?
Metabolic process that occurs in the cytosol resulting in the conversion of acetyl-CoA to fatty acids
A decrease in fatty acid synthase activity is likely to INCREASE levels of cytosoilic acetyl-CoA
De Novo Lipogenesis (Fatty Acid Synthesis)
Process by which fatty acids are built from scratch in the cytoplasm of fat cells (adipocytes) or liver cells (hepatocytes)
16-carbon fatty acids (Palmitate) are produced from the condensation of acetyl-CoA precursor molecues
The process is anabolic and requires NRG and reducing power to facilitate the condensation
This reducing power comes from the reducing agent NADPH
Acetylation Regulates DNA Structure
DNA interacts with histones due to the attraction between the negatively charged DNA backbone and hte positively charged resiudes on histones
The positively charged histone residues can be acetylated by histone acetyltransferase enzymes which reduces the attraction between histones and DNA thus REDUCING the coiling and packing of DNA around histones
Euchromatin has acetylated lysines which facilitates increased gene expression as they are loosely packed
Heterochromatin has deacetylated lysines which encourages strong packing between DNA nad histones thus DECREASING gene expression
Describe the citric acid/Kreb’s/TCA cycle step by step
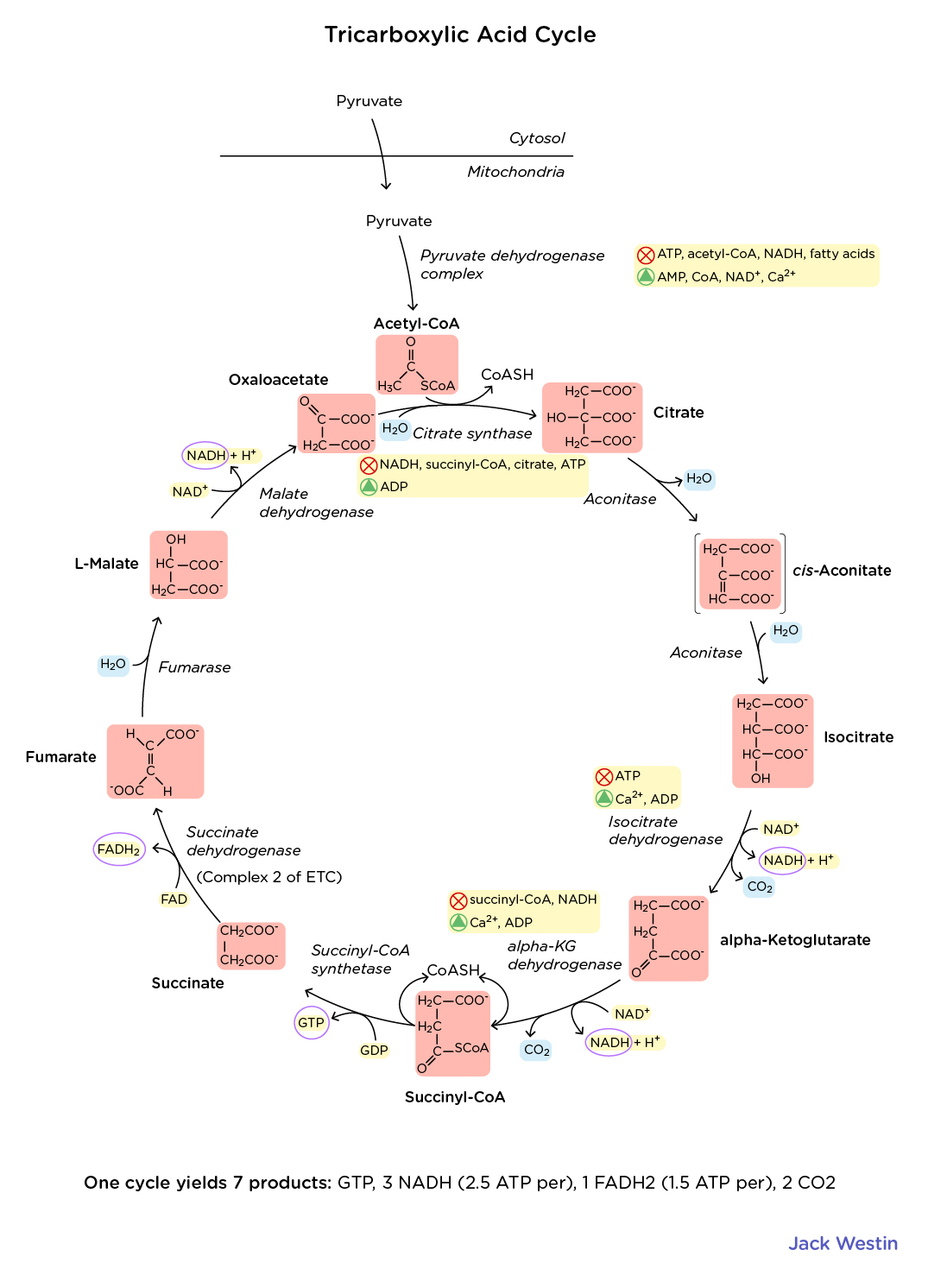
Know the structures of each part of glycolysis and the Kreb’s Cycle
If you know the NAMES of the intermediates, you should inherently have a basic familiarity with the structures too.
Just enough to be able to recognize them, if asked "which TCA Cycle intermediate is shown below". More than that is probably quite unnecessary.
Knowing the carbon positions in TCA cycle intermediates helps track metabolic fates of amino acids. Here's a structured breakdown:
Key Carbon Positions in TCA Intermediates
Oxaloacetate (OAA) → C1 (carbonyl), C3 & C4 (other substituents)
Succinate → C1 & C4 (carbons for functional groups, symmetric molecule)
Citrate → C3 (-OH)
Isocitrate → C3 (-OH) but now moved via isomerization
Amino Acid Integration with TCA
Glucogenic Entry Points:
OAA (C1, C3, C4) → Aspartate, Asparagine
α-Ketoglutarate (C2, C3, C4, C5) → Glutamate, Glutamine, Proline, Arginine, Histidine
Succinyl-CoA (C1, C4, precursor to succinate) → Methionine, Isoleucine, Valine, Threonine
Fumarate (C2, C3, C4, C1) → Phenylalanine, Tyrosine
Ketogenic Entry Points:
Acetyl-CoA → C2 entry into citrate (C3 -OH) → Leucine, Lysine, (partly Isoleucine, Phenylalanine, Tyrosine, Tryptophan)
Using this framework, you can quickly match amino acids to their respective TCA intermediates and track metabolic pathways efficiently.
What are the different methods for isolating elements of things in biochemistry?
ELISA
Uses antibodies to detect the presence of a protein in a liquid sample
Isoelectric Focusing
Separates small macromolecules based on their charge
Centrifugation
Separates particles from a homogenate according to their DENSITY, can isolate parasitic vacuoles from denser cellular components for example
Immunohistochemistry
Technique used to identify the presence of particular antigens in a mixture
Cannot be used to separate components of a homogenate
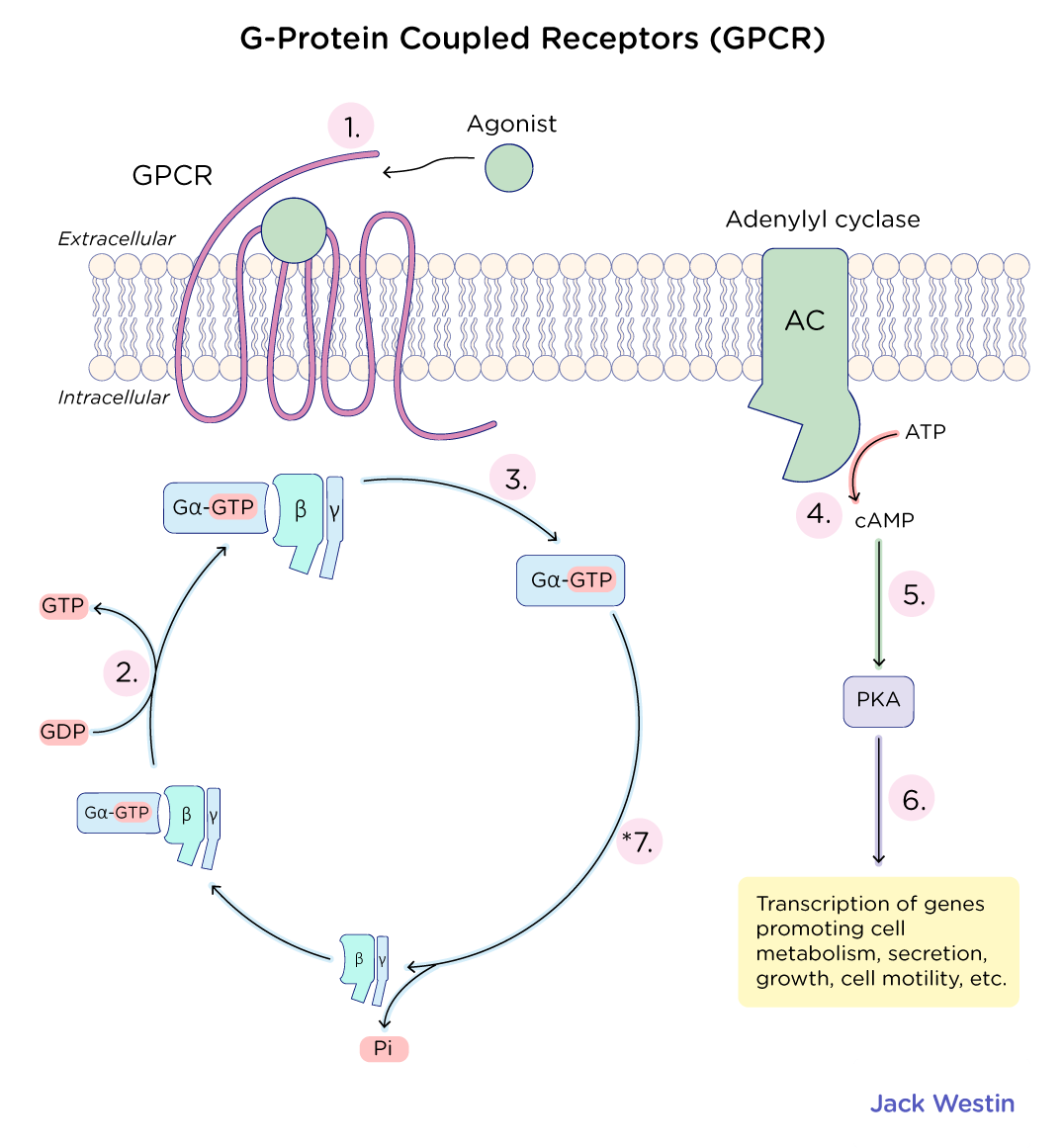
How do GPCRs work?
How to interpret Lineweaver-Burk (double-reciprocal plots)?
G-Protein Coupled Receptors
GPCR Key Takeaways for MCAT Structure & Function
7-transmembrane alpha-helices (hydrophobic exterior, hydrophilic interior)
N-terminus (extracellular) → Ligand binding
C-terminus (intracellular) → Regulation
GPCR Activation
Ligand binds extracellularly causing a conformational change in GPCR
The activation of the G-protein causes α and βγ subunits to dissociate
α-subunit (GDP → GTP exchange) determines signaling pathway
α and βγ interact with target proteins → Signal amplification
Signal termination: α-subunit hydrolyzes GTP → reassembles inactive G-protein
Major Gα Subunit Pathways
Gαs (stimulatory) → BINDS to and activates adenylate cyclase
Adenylate cyclase (AC) is a membrane-bound enzyme that converts ATP → cAMP
cAMP is a second messenger that activates protein kinase A (PKA) → Phosphorylates target proteins → Cellular response
Gαi (inhibitory) → Inhibits adenylate cyclase → ↓ cAMP → Less PKA activation
Gαq → Activates phospholipase C (PLC) → PIP2 → DAG + IP3
IP3 → Releases Ca²⁺ from ER → Second messenger effects
cAMP, after having been converted from ATP by adenylate cyclase goes and ACTIVATES PKA which then goes to phosphorylate target proteins to induce various cellular responses
MCAT Focus: Understand ligand binding, G-protein activation, adenylate cyclase's role in cAMP production, and major signaling pathways!
INCREASED LEVELS OF cAMP activate downstream signaling pathways, leading to a variety of cellular responses
Lineweaver-Burk (double-reciprocal plots)
Useful for plotting effects of inhibitor
1/reaction rate is 1/[substrate]
Y intercept is equal to 1/vmax
X intercept is equal to 1/Km
Noncompetitive inhibitors affect enzymatic activity but do not alter affinity/Km will thus lower VMax and not affect Km
![<p>G-Protein Coupled Receptors</p><p></p><p><strong>GPCR Key Takeaways for MCAT</strong> <strong>Structure & Function</strong></p><ul><li><p><strong>7-transmembrane alpha-helices</strong> (hydrophobic exterior, hydrophilic interior)</p></li><li><p><strong>N-terminus (extracellular)</strong> → Ligand binding</p></li><li><p><strong>C-terminus (intracellular)</strong> → Regulation</p></li></ul><p><strong>GPCR Activation</strong></p><ol><li><p><strong>Ligand binds extracellularly</strong> causing a conformational change in GPCR</p></li><li><p><strong>The activation of the G-protein causes α and βγ subunits to dissociate</strong></p><ul><li><p><strong>α-subunit (GDP → GTP exchange)</strong> determines signaling pathway</p></li><li><p><strong>α and βγ interact with target proteins</strong> → Signal amplification</p></li></ul></li><li><p><strong>Signal termination</strong>: α-subunit hydrolyzes GTP → reassembles inactive G-protein</p></li></ol><p><strong>Major Gα Subunit Pathways</strong></p><ul><li><p><strong>Gαs (stimulatory)</strong> → BINDS to and activates <strong>adenylate cyclase</strong></p><ul><li><p><strong>Adenylate cyclase (AC)</strong> is a membrane-bound enzyme that converts <strong>ATP → cAMP</strong></p></li><li><p><strong>cAMP</strong> is a second messenger that activates <strong>protein kinase A (PKA)</strong> → Phosphorylates target proteins → Cellular response</p></li></ul></li><li><p><strong>Gαi (inhibitory)</strong> → Inhibits <strong>adenylate cyclase</strong> → ↓ cAMP → Less PKA activation</p></li><li><p><strong>Gαq</strong> → Activates <strong>phospholipase C (PLC)</strong> → <strong>PIP2 → DAG + IP3</strong></p><ul><li><p><strong>IP3</strong> → Releases Ca²⁺ from ER → Second messenger effects</p></li></ul></li></ul><p></p><ol start="4"><li><p>cAMP, after having been converted from ATP by adenylate cyclase goes and ACTIVATES PKA which then goes to phosphorylate target proteins to induce various cellular responses</p></li></ol><p></p><p><strong>MCAT Focus:</strong> Understand ligand binding, G-protein activation, <strong>adenylate cyclase's role in cAMP production</strong>, and major signaling pathways!</p><p></p><p>INCREASED LEVELS OF cAMP activate downstream signaling pathways, leading to a variety of cellular responses</p><p><strong><u>Lineweaver-Burk (double-reciprocal plots)</u></strong></p><ul><li><p>Useful for plotting effects of inhibitor</p></li><li><p>1/reaction rate is 1/[substrate]</p></li><li><p>Y intercept is equal to 1/vmax</p></li><li><p>X intercept is equal to 1/Km</p></li><li><p>Noncompetitive inhibitors affect enzymatic activity but do not alter affinity/Km will thus lower VMax and not affect Km</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/0618ca1a-2c8b-457b-97c3-a4869acc4996.png)