Chem Lab- Science Olympiad
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
29 Terms
What is the formula for Molarity?
moles of solute/liters of solution
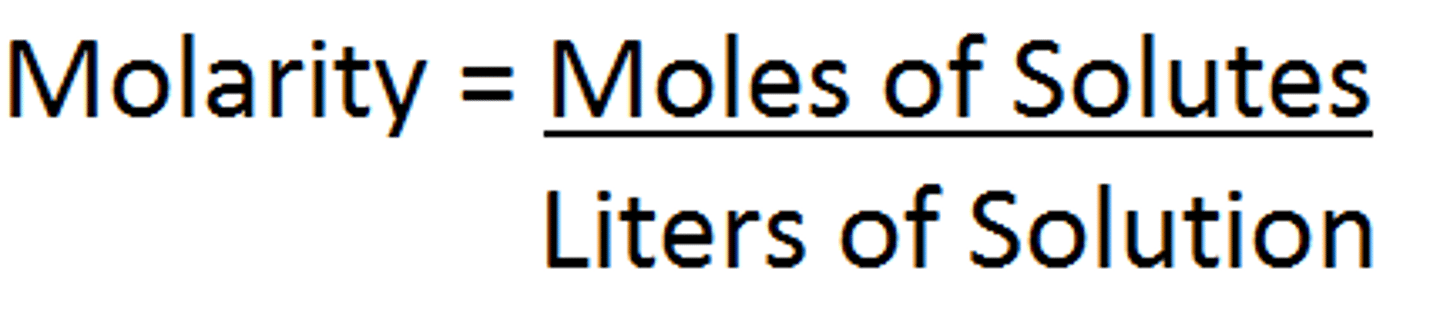
What is an acid?
An acid is a source of hydrogen ions, H⁺, has a pH less than 7
Strong bases
NaOH, KOH, Ca(OH)2, Mg(OH)2
What are physical properties?
characteristics of a substance that can be observed without changing it into another substance
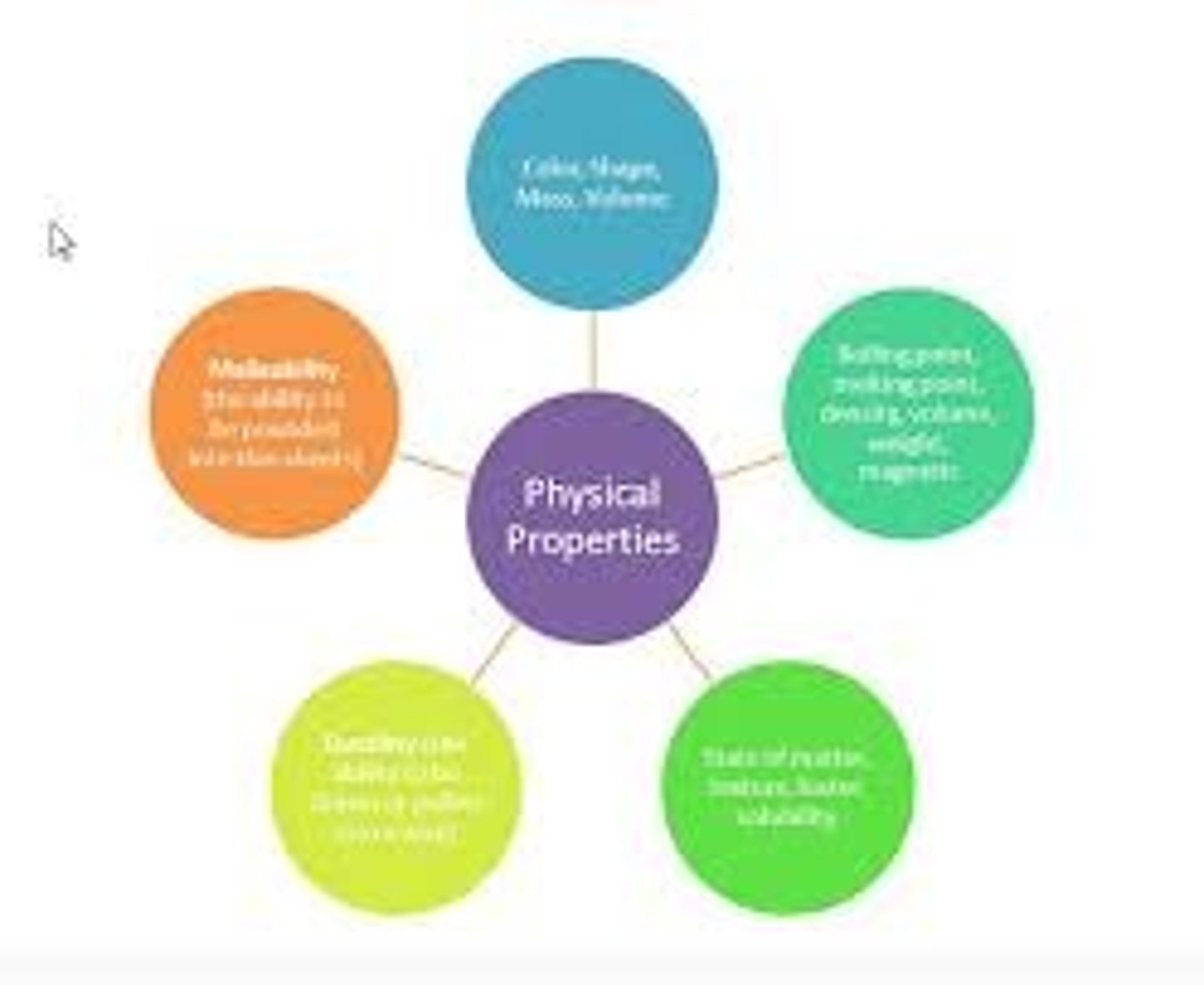
What are examples of physical properties
viscosity, conductivity, malleability, hardness, melting point, boiling point, and density

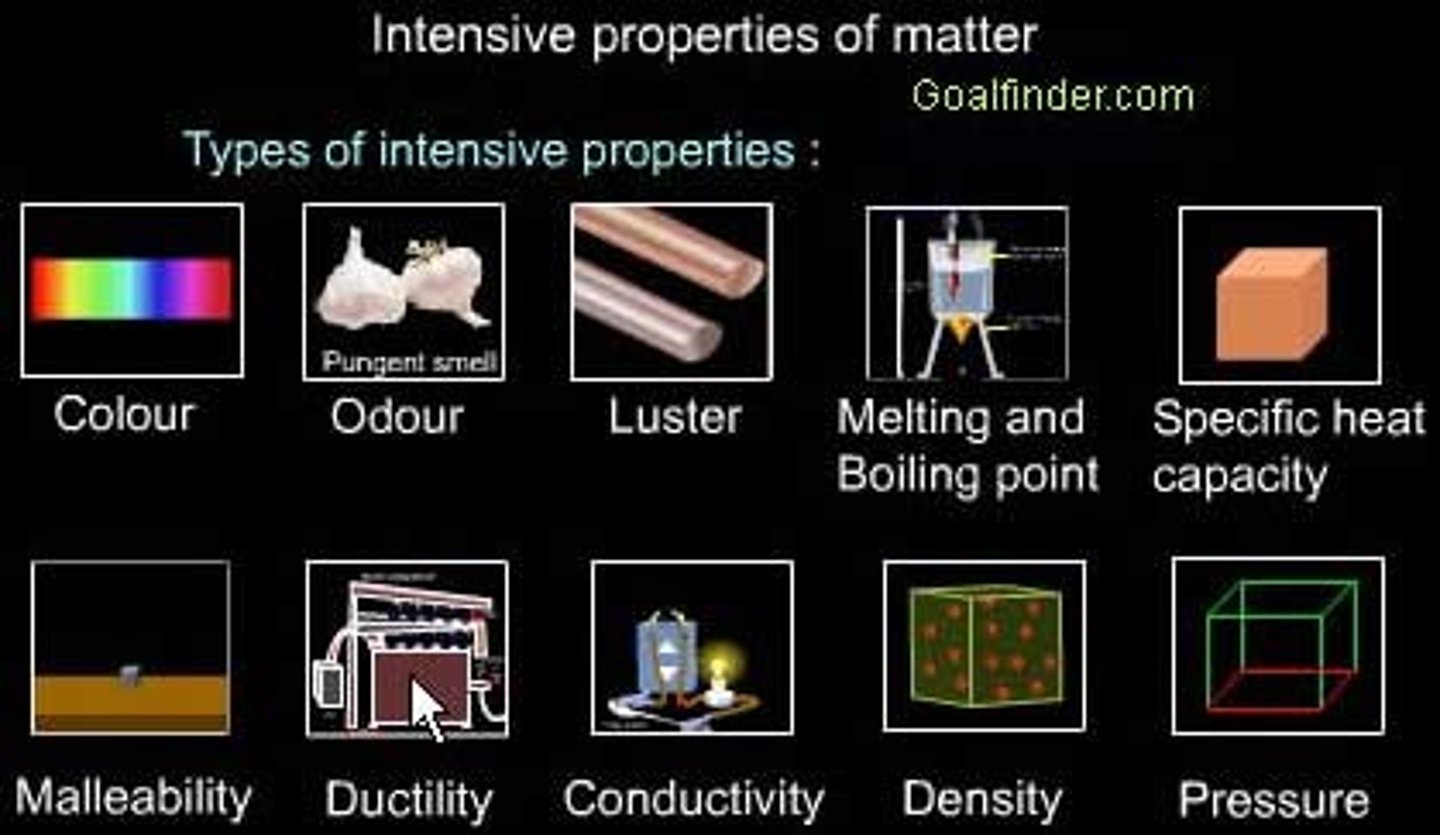
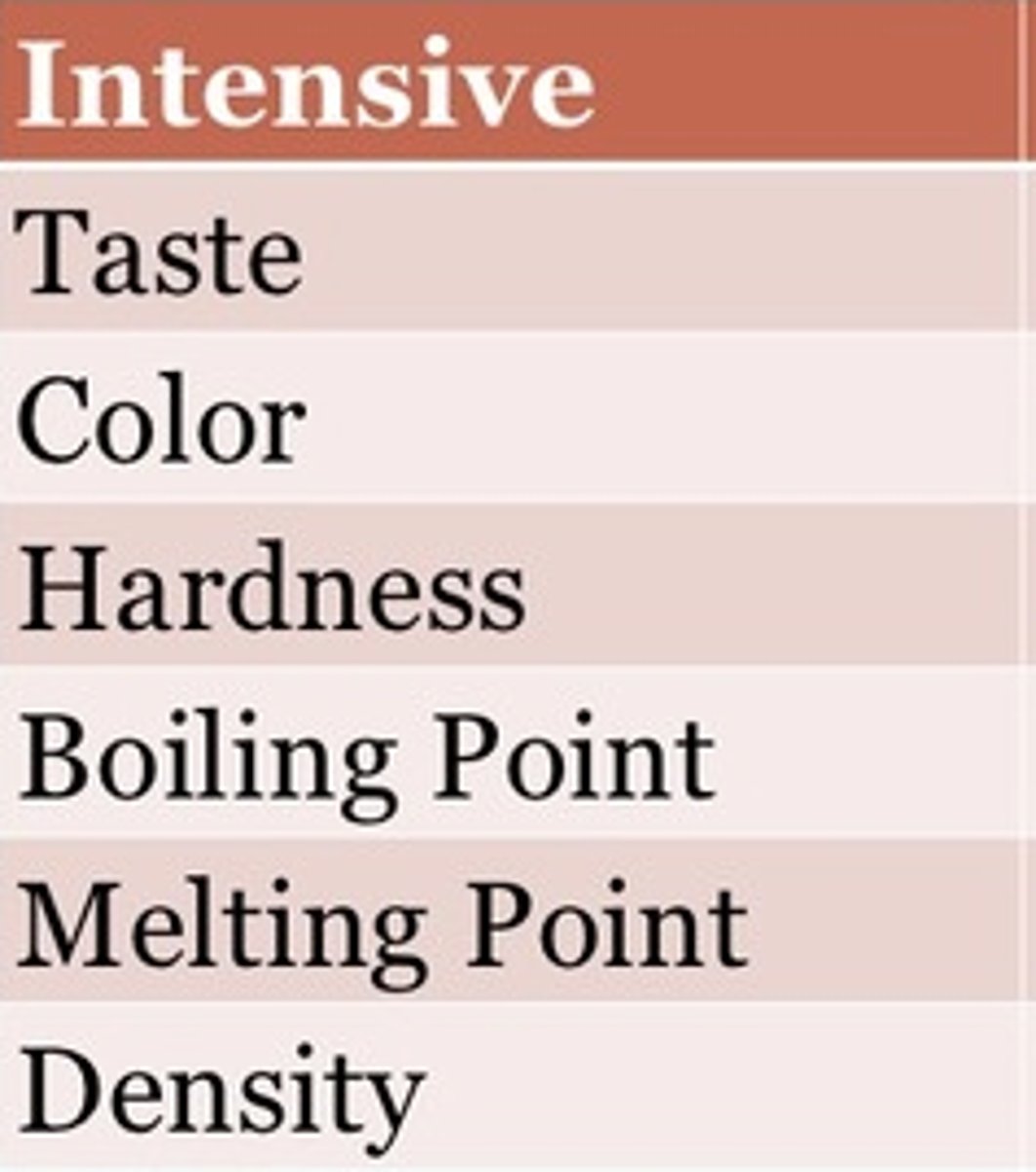
What are intensive properties?
properties that do not depend on the amount of matter present
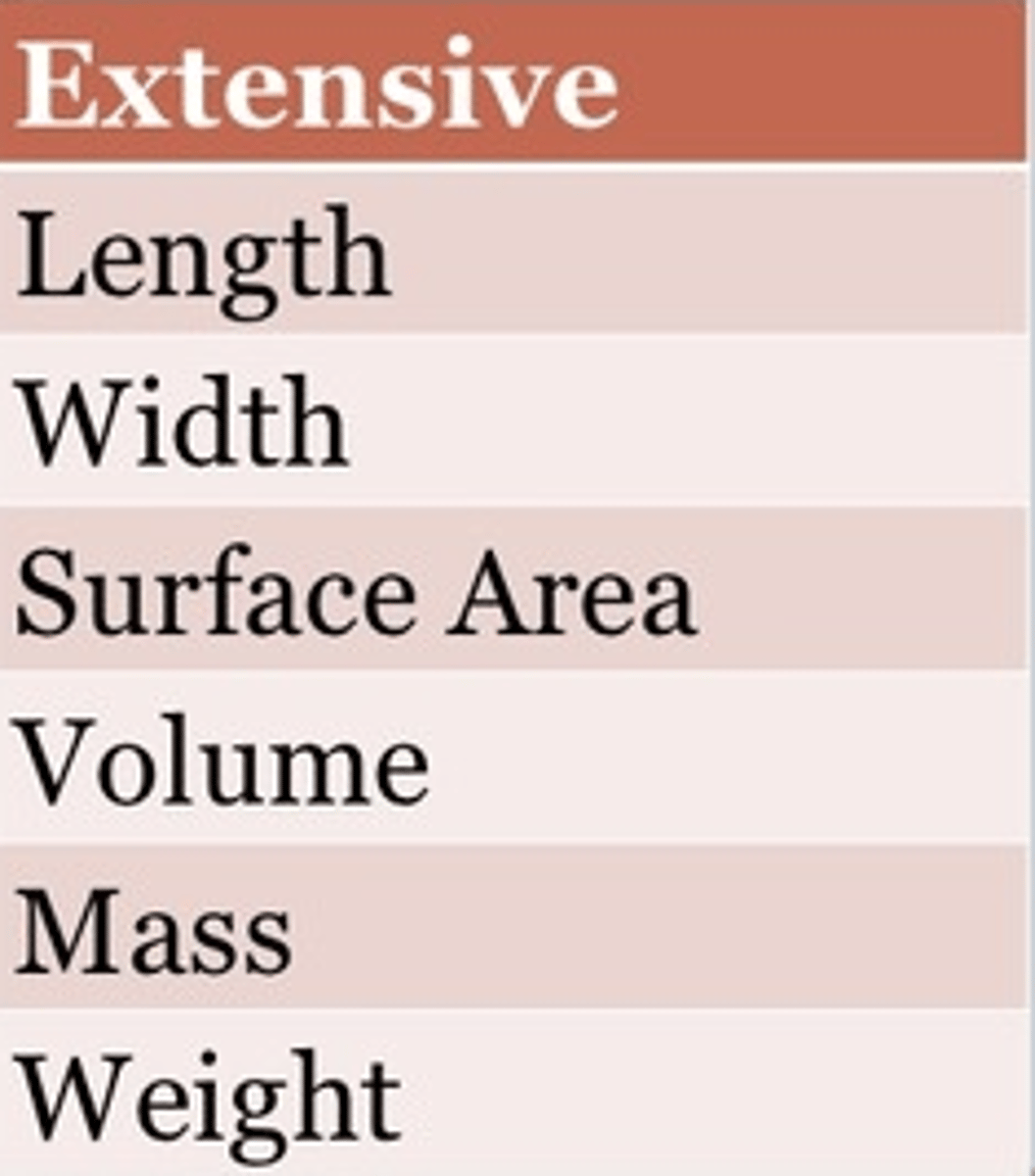
What are extensive properties?
properties that depend on the amount of matter that is present

What are examples of extensive properties?
mass, length, volume, solubility
What are examples of intensive properties?
Conductivity Density Color Temperature Resistance Magnetism
What is a base?
A substance with a pH greater than 7, accept H+ ions, donate e-, or give off OH- in H20
What is an aqueous solution?
a solution in which water is the solvent
Examples of aqueous solutions
Saltwater, Cola, rain, acid solutions
Exothermic
Chemical Reaction in which energy is primarily given off in the form of heat
Endothermic
(of a chemical reaction or compound) occurring or formed with absorption of heat

What happens when acetic acid, a weak electrolyte, dissolves in water?
Hydronium ions form, The solution will conduct electricity, and most of acid remains as non ionized molecules in equilibrium with ions.
The stronger the acid.... _____
the weaker its conjugate base is.

The weaker the acid....._____
the stronger its conjugate base is.
What is the pH for acids?
Less than 7
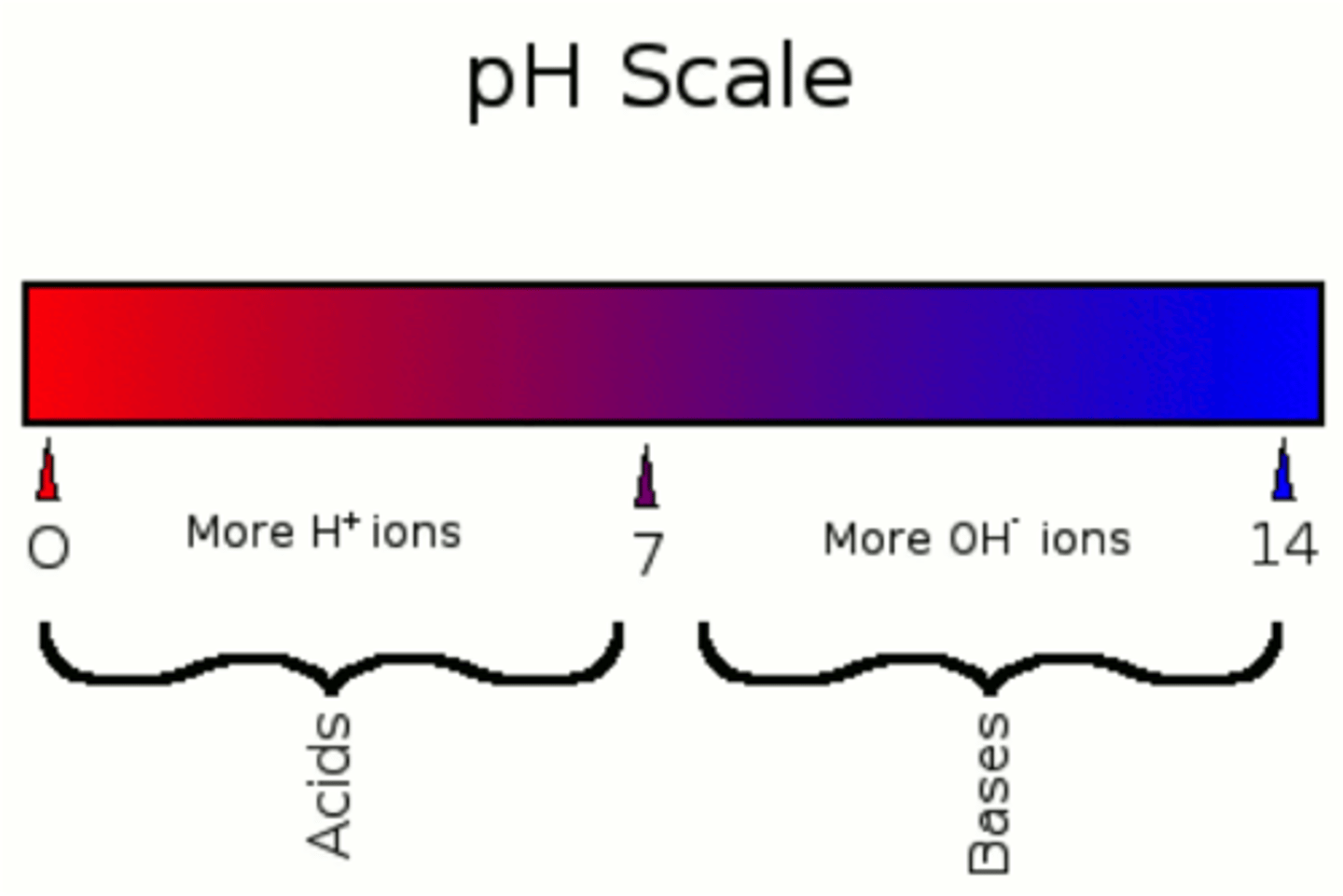
What is the pH for bases
8-14
What are examples of acids?
apple juice, vinegar, lemon juice, etc.
What are examples of bases?
ammonia, shampoo, baking soda
What is an example of a neutral on the pH scale?
Distilled Water
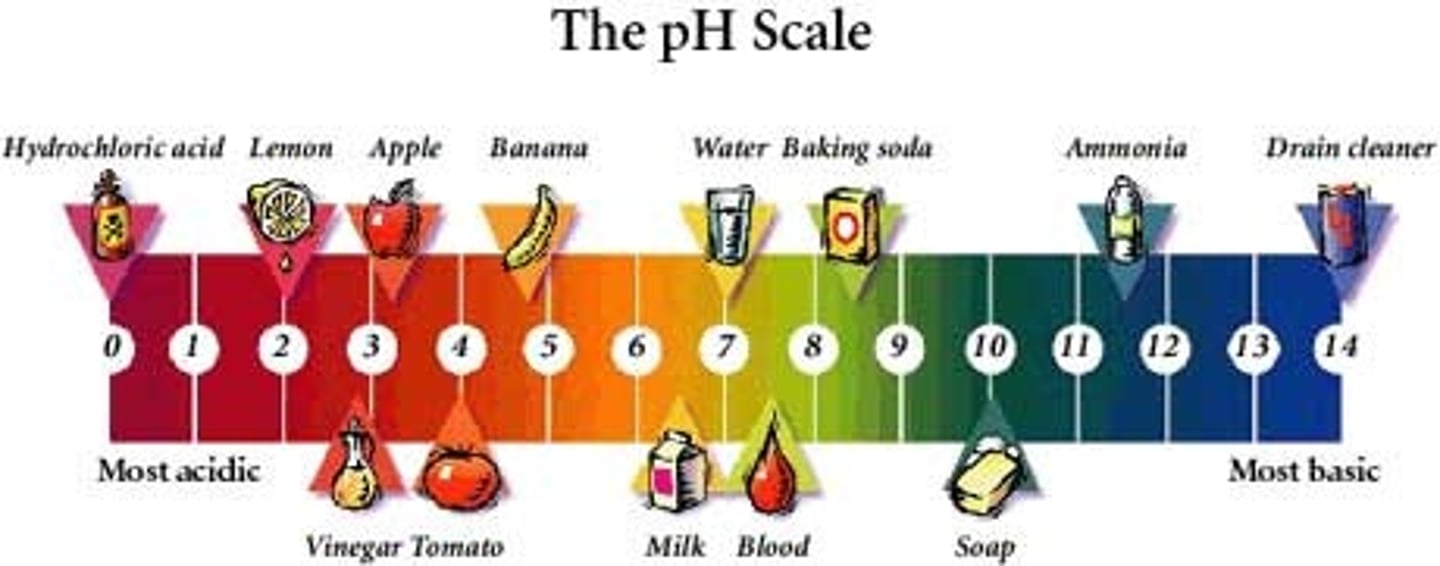
The concentration of acetic acid (pKa = 4.75) in vinegar is about 1.0 M. With this information, what do you predict the pH of vinegar to be?
2.4
Which substance, when dissolved in water, forms a solution that conducts an electric current?
CH3COOH
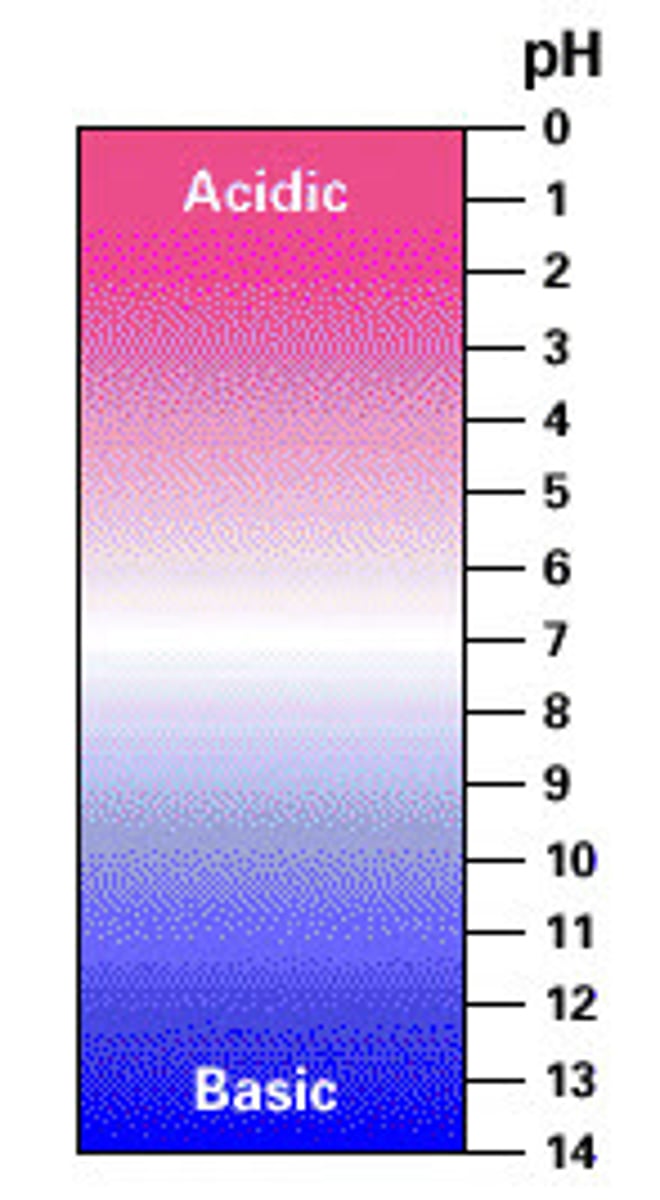
Which pH indicates the highest concentration of H3O + ions?
1
What is the pH of pure water/neutral?
7
Soluble
If two liquids are mixed and do not separate upon standing

Insoluble
incapable of being dissolved

chemical property
A characteristic of a pure substance that describes its ability to change into different substances
