DISSOLVING
1/29
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Solution
A homogenous mixture of 2 or more substances, formed when a solute dissolves in a solvent.
Dissolve
When a solute is mixed with a solvent to form a solution
Two layer solution
A two-layer solution, also called an immiscible mixture, forms when two liquids that do not dissolve in each other are combined. These liquids have different densities and do not mix due to differences in their molecular properties, such as polarity.
A two-layer solution forms when two liquids that don’t mix, like oil and water, are combined. The liquid with lower density (oil) floats on top of the denser one (water), creating two distinct layers.
Examples of two layer solutions
1. Oil and Water: A classic example where oil floats on water due to differences in density and polarity.
2. Vinegar and Olive Oil: Common in salad dressings, these two liquids form separate layers when left undisturbed.
3. Gasoline and Water: Gasoline, being less dense and non-polar, floats on water.
4. Kerosene and Water: Kerosene, like other hydrocarbons, does not mix with water and forms a top layer.
5. Mercury and Water: Mercury, a metal in liquid form, sinks to the bottom due to its high density, forming two layers with water.
Insoluble
A substance that does not dissolve in a given solvent
Soluble
A substance that will dissolve in a given solvent
Solute
The substance that dissolves in a solvent to make a solution
Solvent
The substance in which a solute dissolves in
Transparent
Solutions are transparent, this means you can see through them
Colored
Solutions can be colored.
Opaqueness
A liquid that is not transparent but opaque showing that it is not a solution
Melting vs dissolving
- Melting occurs when a solid turns into a liquid due to heat.
- Dissolving happens when a solid (solute) breaks down and mixes with a liquid (solvent) to form a solution.
In melting, only heat is involved, while dissolving involves the interaction between two substances.
Dilute
A solution that has less solute particles compared to solvent particles
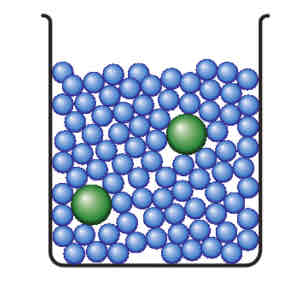
Concentrated
A solution that has less solvent particles compared to solute particles
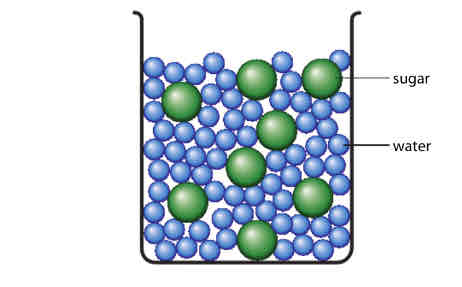
Saturated Solute
A solution in which no more solute can dissolve at a given temperature
Temperature n solubility
Most solute dissolves quick and easily in hot water than cold water because the particles have more energy, they vibrate and move quickly.
The temperature increases as the solubility of most salt increases
Working out concentration
1g/10cm3
1/10=0.1g/cm cubed
Other solvents
1. Ethanol – Used in cleaning, fuels, and beverages.
2. Acetone – Found in nail polish remover and cleaning products.
3. Benzene – Used in industrial processes (though toxic).
4. Toluene – Common in paint thinners and adhesives.
5. Methanol – Used in antifreeze and fuel.
6. Hexane – Employed in oil extraction and cleaning.
7. Chloroform – Used in laboratory applications and as a solvent in industries.
Variables
Factors that can be changed in an investigation
Dependent variable
The factors that change in an investigation as a result of changing the independent variable
Independent variable
The factor that is changed by the experimenter in an investigation
Controlled variable
The variables that you keep the same
Interval
The difference between two things used for the changes in the independent variable
Range
The difference between the highest and lowest values
Real life applications of solubility
1. Medicine: Drugs need to dissolve in your body to work properly.
2. Water Treatment: Solubility helps remove harmful substances from water.
3. Cooking: Ingredients like sugar and salt dissolve to mix into food.
4. Aquatic Life: Fish need oxygen dissolved in water to survive.
5. Cleaning: Soaps and detergents dissolve dirt and grease.
6. Manufacturing: Factories rely on solubility for efficient chemical reactions.
7. Cosmetics: Skincare products depend on how ingredients dissolve to absorb better into the skin.
Chomotography
“Chroma” intensity of color “grapein” means to write
Paper Chomotagraphy
A seperation technique used to seperate mixtures of soluble substances with different colors
Real life applications of paper chomotography
1. Food testing: To detect additives, contaminants, or artificial colours in food and drinks.
2. Drug testing: Used in sports or medicine to identify substances in blood or urine samples.
3. Forensics: To analyse ink, dyes, or chemicals at crime scenes.
4. Environmental testing: To check for pollutants in water, air, or soil.
5. Pharmaceuticals: To ensure the purity of medicines and separate compounds during drug development.
6. Plant studies: To identify pigments in leaves or flowers.
Advantages of paper chomotography
1. Simple and easy to use: It requires minimal equipment and is easy to set up.
2. Cost-effective: It is inexpensive compared to other separation techniques.
3. Portable: It can be performed anywhere, even outside a laboratory.
4. Efficient for small samples: Only a tiny amount of the mixture is needed.
5. Separates complex mixtures: It effectively separates multiple substances in a mixture.
6. Non-destructive: The components remain intact after separation.
7. Versatile: Can be used for a wide range of substances like pigments, dyes, and amino acids.
Disadvantages of paper chomotography
1. Limited accuracy: It is less precise compared to advanced techniques.
2. Not suitable for all substances: It only works for mixtures that dissolve in the solvent.
3. Slower process: Separation can take longer compared to other methods.
4. Small sample capacity: It cannot handle large or concentrated samples effectively.
5. Low sensitivity: It may not detect very small quantities of substances.
6. Poor separation of similar compounds: Substances with similar properties may not separate clearly.