Ch.6
6.1: The Wave Nature of Light
Visible light is a type of electromagnetic radiation or radiant energy
Speed of light is 2.998 × 10^8
All electromagnetic radiation move at the speed of light
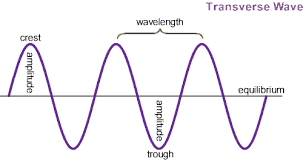
The number of wavelengths (distance between crest/trough and the next crest/trough) completed is the frequency
6.2: Quantized Energy and Photon
Blackbody radiation is the emission of light from hot objects
Photoelectric effect is the emission of electrons from a metal surfaces on which light shines
- Photon is a packet of energy
- Energy of photon = E(energy)=h(planks constant)v(frequency)
Emission spectra is the emission if light from electronically excited gas atoms
E(energy)=h(planks constant)v(frequency)
- Planck constant is 6.26 * 10^-34
Planck’s theory states that matter can only absorb or emit heat in the multiples of hv
A photon has work function energy (minimum energy needed to kick off an electron)
Increasing the intensity of light does not lead to the emission of electrons; only increasing the frequency can kick off electrons.
- Increasing the intensity only increases the number of photons hitting the surface at a time; does not increase the energy of each photon
- When frequency increases than work function, electrons are emitted
6.3: Line Spectra and the Bohr Model
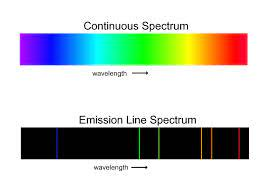
A spectrum containing radiation of only one specific wavelength is called line spectrum
Bohr’s Model:
Only orbits of a certain radii are permitted fir the electron in a hydrogen atom
An electron permitted orbit is in an allowed energy state
- An electron in an allowed energy state does not radiate energy
Energy is emitted or absorbed by the electron only as the electron changes from one allowed energy level to another
Lower the energy, the more stable the atom is
- Lowest energy state is called the ground state
- When the electron is in an higher- energy sate the electron is in an excited state.
When △E is positive the photon is absorbed
*Bohr model only works for hydrogen *
Two main takeaways of the Bohr model:
1. electrons exist only in certain discrete energy levels
- energy is involved in the transition of an electron from one level to another
6.4: The Wave Behavior of Matter
The radiation can either have a wave like behavior or a particle like behavior
An electron moving about the nucleus of an electron behaves like a wave and therefore has a wavelength
- wavelength=h/mv
- this applies to all matter
- Hinesburg principle is also called the uncertainty principle which states that the dual nature of matter (wave and particle like behaviors) places of the limitation of how precisely we can calculate both the location and momentum of an object, in this instance electron
6.5: Quantum Mechanics and Atomic Orbitals
wave functions describe the electron in an atom
probability density or the electron density is the probability that an electron will be found at that location
each orbital has a characteristic shape and energy
6.6: Representations of Orbitals
s orbital is the lowest energy orbital-- it is spherically symmetrical-1
- in an ns orbital number of peaks = n
- number of nodes = n-1
- as n increases the probability of finding an electron there also increases
p orbital-3
- 3 sublevels
- not symmetrical
d and f orbitals-5
- 5 d orbitals
6.7: Many Electron Atoms
Electron spin: causes each electron to spin individually around its axis
- Electrons must have opposite spins
6.8: Electron Configurations
- The way electrons are distributed is electron configuration
- stable= ground state or when electrons are in the lowest energy level
- There can be at most 2 electrons in a single orbit
- Paired electrons are electrons with opposite spins
- Unpaired electrons cause paramagnetic and do not have another electron with an opposite spin.
6.9: Electron Configurations and the Periodic Table
Electron configurations correlate to their location on the periodic table
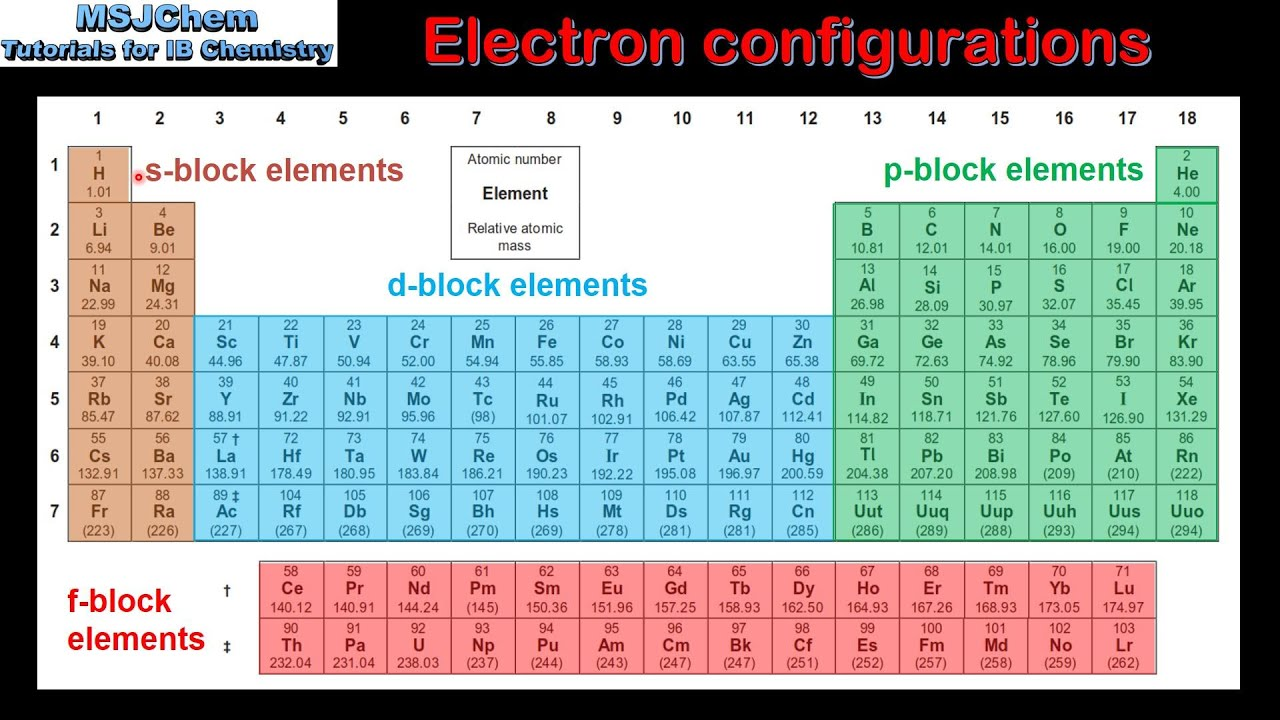
4s is filled before 3d
Can write the noble gas before in [ ]
half full shell is more stable; full shell is the most stable