Chemical analysis
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What is a pure substance?
A single element or a single compound
Not mixed with any other substance
Key feature of pure substances
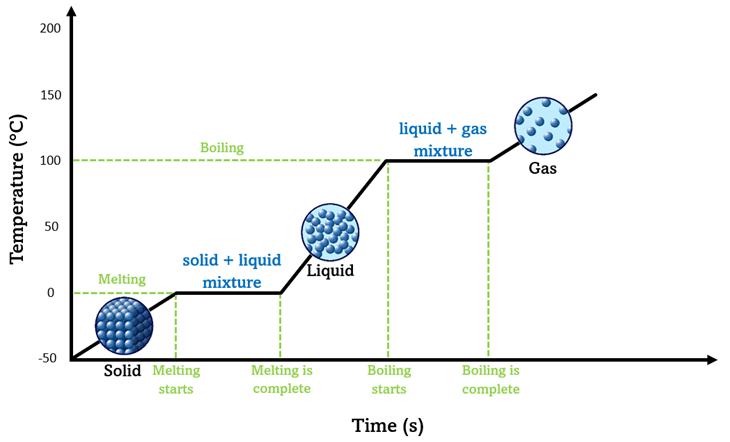
Melts at a specific fixed boiling point
Specific fixed boiling point
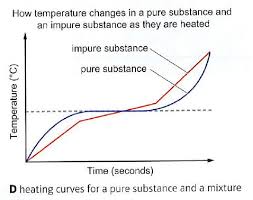
Key feature of impure substances
Melt and boil over a range of temperatures
How do we use the melting and boiling point on a graph to find if a substance is pure?
Goes up in fixed amounts
What does a impure substances graph look like?
What is a formulation?
A complex mixture that has been designed as a useful product
Each component is carefully measured to be exactly what’s needed
What does paper chromatography allow us to do?
Separate substances based on their different solubilities
What are the stages to chromatography?
Take a piece of chromatography paper and drew a pencil line near the bottom
Put a dot of the first solution onto the line and a dot of the second solution
Place the paper in a solvent (dissolves substances)
The solvent makes its way up the paper and carries the dots up the paper
What is the stationary phase?
The stationary phase because it does not move
What is the mobile phase?
The solvent because it moves
What do the results show?
If the dot of solution remains one dot it is a pure substance
If the dot of solution becomes more then one dot it is a mixture
Why does paper chromatography work?
Different substance have different solubilities
Why is the starting line drawn in pencil?
The pen ink would move up the paper
How can paper chromatography be used to identify a unknow substance?
Follow the usual steps
Find where the solvent moved to and where the dot moved
Measure the distance moved by the unknown chemical (pencil line to dot)
Measure the distance moved by the solvent (pencil line to solvent dot)
Calculate the Rf
Compare this to the database
What is the equation for the Rf?
Rf = distance moved by substance / distance moved by solvent
What are the problems with finding unknow solvents?
Several different substances could have the same Rf value
If its never been analysed before, then there will not be an Rf value
What is the test for hydrogen gas?
Insert burning split into gas
Hydrogen burns rapidly and produces a pop sound
What is the test for oxygen gas?
Insert a glowing splint into the gas
It is oxygen if it relights
What is the test for carbon dioxide gas?
Draw some of the gas into a plastic pipette
Bubble the gas through limewater
Repeat several times
If its carbon dioxide it turns cloudy
What is limewater?
An aqueous solution of calcium hydroxide
What is the test for chlorine gas?
Insert damp litmus paper into the gas
Chlorine bleaches the litmus paper and turns it white