The Periodic Table
0.0(0)
Card Sorting
1/44
Earn XP
Description and Tags
Last updated 12:52 PM on 11/10/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
1
New cards
Group 1
Alkali metals.
2
New cards
Group 2
Alkaline earth metals
3
New cards
Groups 3-12
transition metals
4
New cards
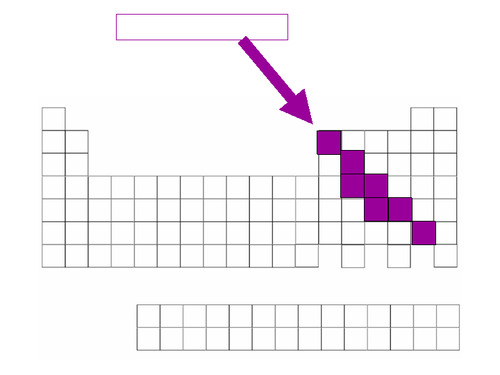
metalloids
Found along the 'staircase'. Have properties of both metals and nonmetals
5
New cards
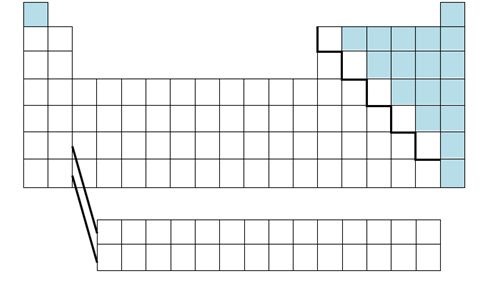
nonmetals
brittle, dull, poor conductors of heat and electricity
6
New cards
Proton
Positively charged particle in the nucleus of an atom

7
New cards
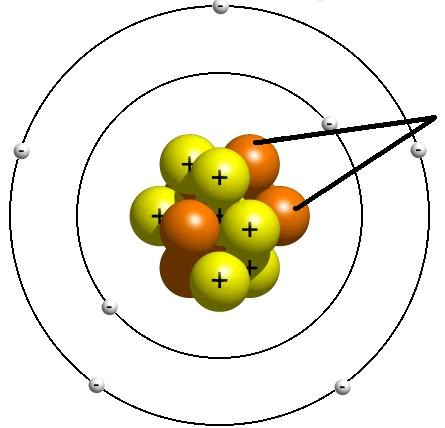
Neutron
A subatomic particle that is neutral and that is found in the nucleus of an atom
8
New cards
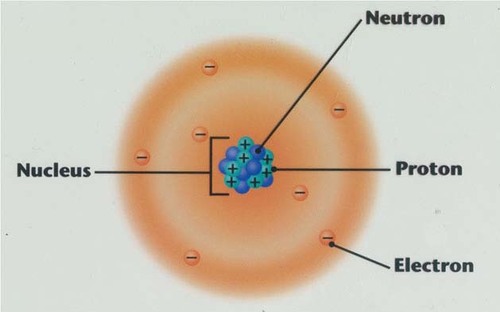
Electron
A tiny, negatively charged particle that moves around the nucleus of an atom.
9
New cards
Atomic Number
The number of protons in the nucleus of an atom of an element

10
New cards
Mass Number
The total number of protons and neutrons in an atom's nucleus

11
New cards
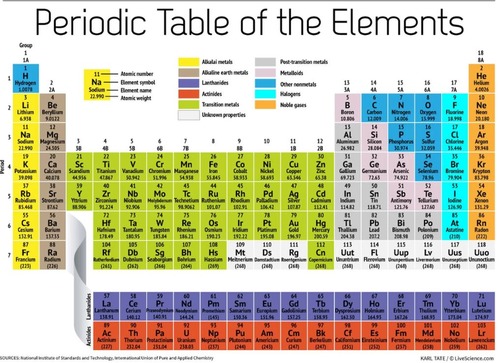
Periodic table
A chart of all chemical elements currently known, organized by atomic number.
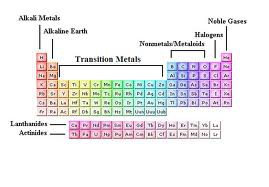
12
New cards
Element
pure substance that consists entirely of one type of atom
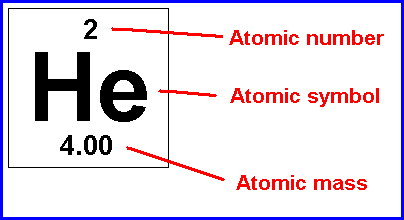
13
New cards
atom
the smallest unit of an element that maintains the properties of that element
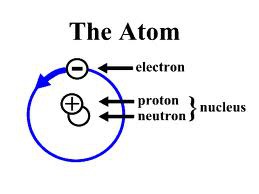
14
New cards
periods
horizontal rows on the periodic table
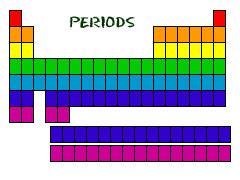
15
New cards
groups
vertical columns on the periodic table
16
New cards
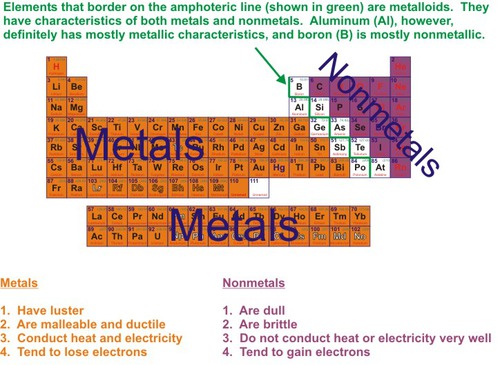
metal
Good conductor of heat and electricity. Has luster and high density
17
New cards
shiny
reflecting light; metallic luster

18
New cards
malleable
physical property of metals; able to be hammered into thin sheets

19
New cards
conductivity
the ability of an object to transfer heat or electricity to another object
20
New cards
reactivity
the ease and speed with which an element combines or reacts with other elements and compounds
21
New cards
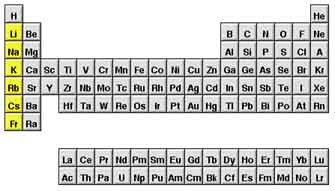
alkali metals
very reactive, not found alone in nature, potassium and sodium are examples
22
New cards
alkaline earth metals
hard, grey-white, good conductors of electricity, calcium and magnesium are examples
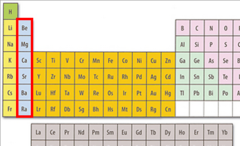
23
New cards
Transition metals
most are hard and shiny, less reactive, examples are iron, copper, nickel and gold
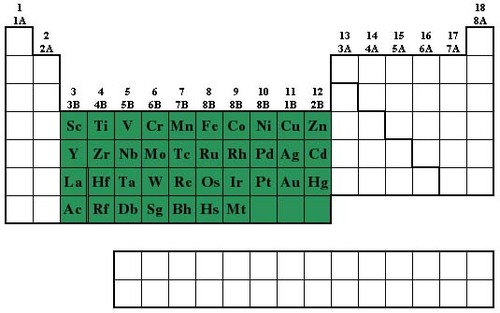
24
New cards
synthetic elements
not found naturally on earth, all elements higher than 92

25
New cards
Particles
Everything in the universe is made of quarks, leptons and force carrier ________________
26
New cards
nucleus
Positively charged, dense center of an atom that contains protons and neutrons
27
New cards
hydrocarbon
Organic compound composed of only carbon and hydrogen
28
New cards
Amu
atomic mass unit; protons and neutrons have a mass of 1 _______
29
New cards
covalent
Type of bond in which atoms share electrons
30
New cards
valence
Electrons on the outermost energy level of an atom
31
New cards
ductile
can be made into a wire
32
New cards
Six
Number of known quarks; also number of periods in a regular school day.
33
New cards
Bonding
Process of atoms joining to form molecules
34
New cards
Amino acids
Organic molecules that contain Nitrogen and are essential part of iving cells
35
New cards
Quark
protons and neutrons are composed of smaller particles called ______________
36
New cards
alcohol
a type of liquid that is flammable and floats on water
37
New cards
isotope
An atom with the same number of protons but a different number of neutrons
38
New cards
Ionization
the process of removing electrons to form ions
39
New cards
atom
smallest part of an element that can take part in a chemical reaction
40
New cards
mass
The nucleus has almost all of the _________ of an atom.
41
New cards
nonmetal
Brittle, poor conductors, no luster; can be a solid (s), liquid (l) or gas.(g)
42
New cards
leptons
The six ________________ consist of the electron, muon, tau and 3 types of neutrinos
43
New cards
S, P, O, N, C, H
The six biologically important elements.
44
New cards
ionic
Type of bonding where an atom gains or loses electrons to form an ion.(+/-)
45
New cards
metallic bond
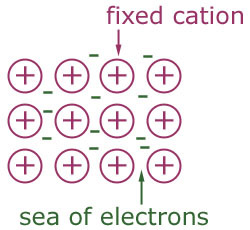
type of bonding where electrons are shared around positive metal ions in a "sea of electrons"