Organic Chemsitry Functional Groups
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
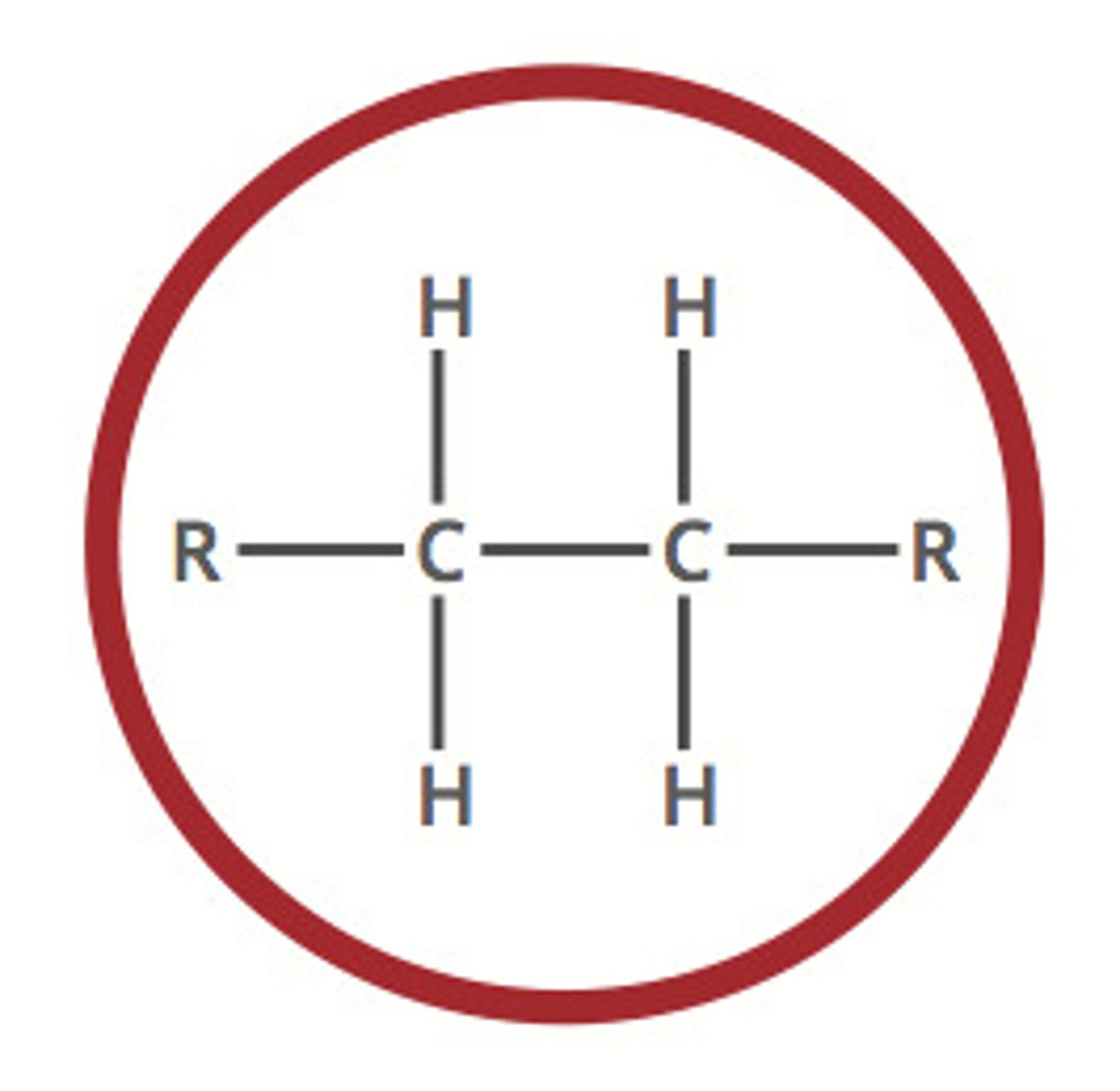
alkane (-ane)
a hydrocarbon containing only single covalent bonds
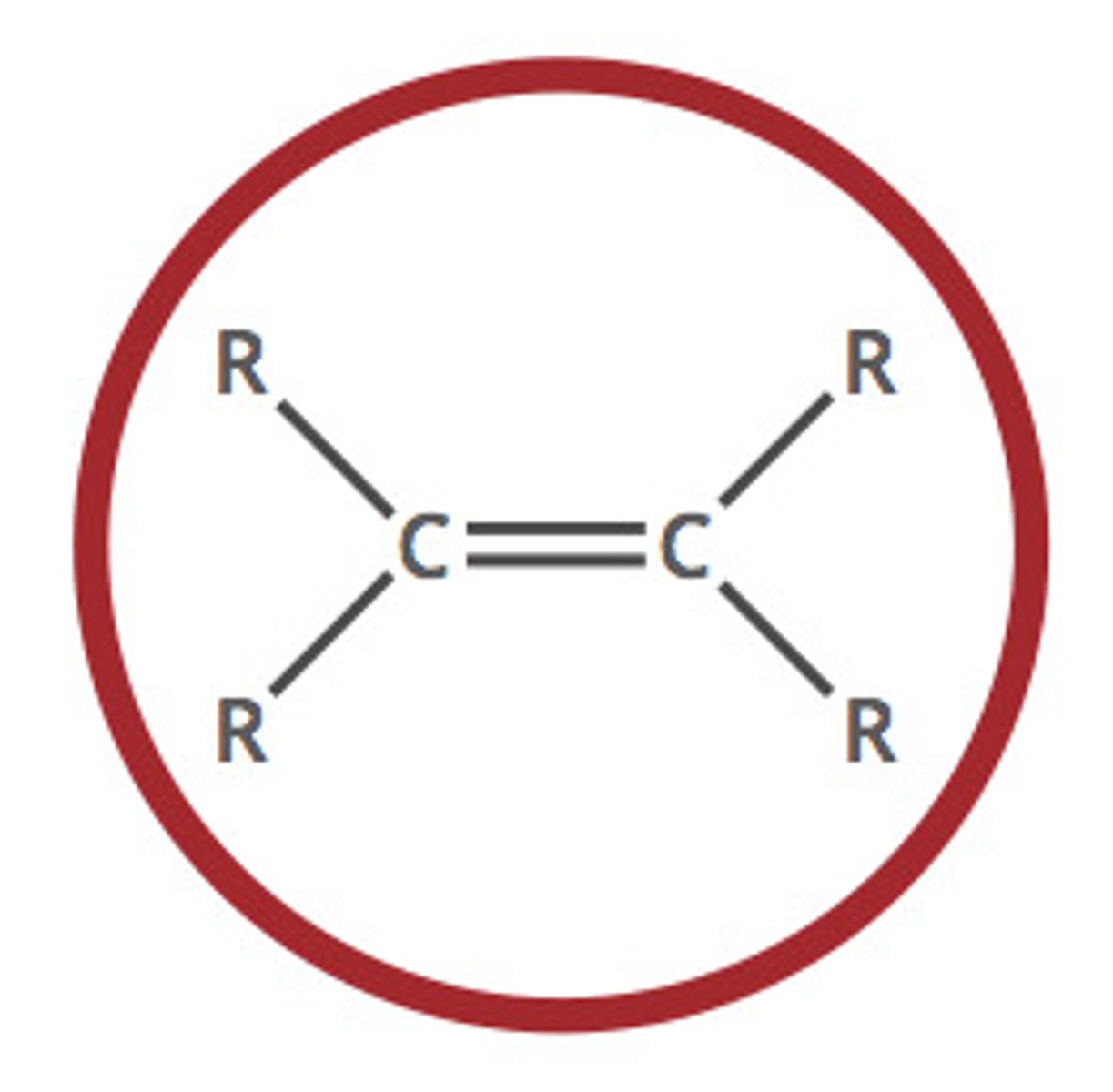
alkene (-ene)
a hydrocarbon containing a double bond
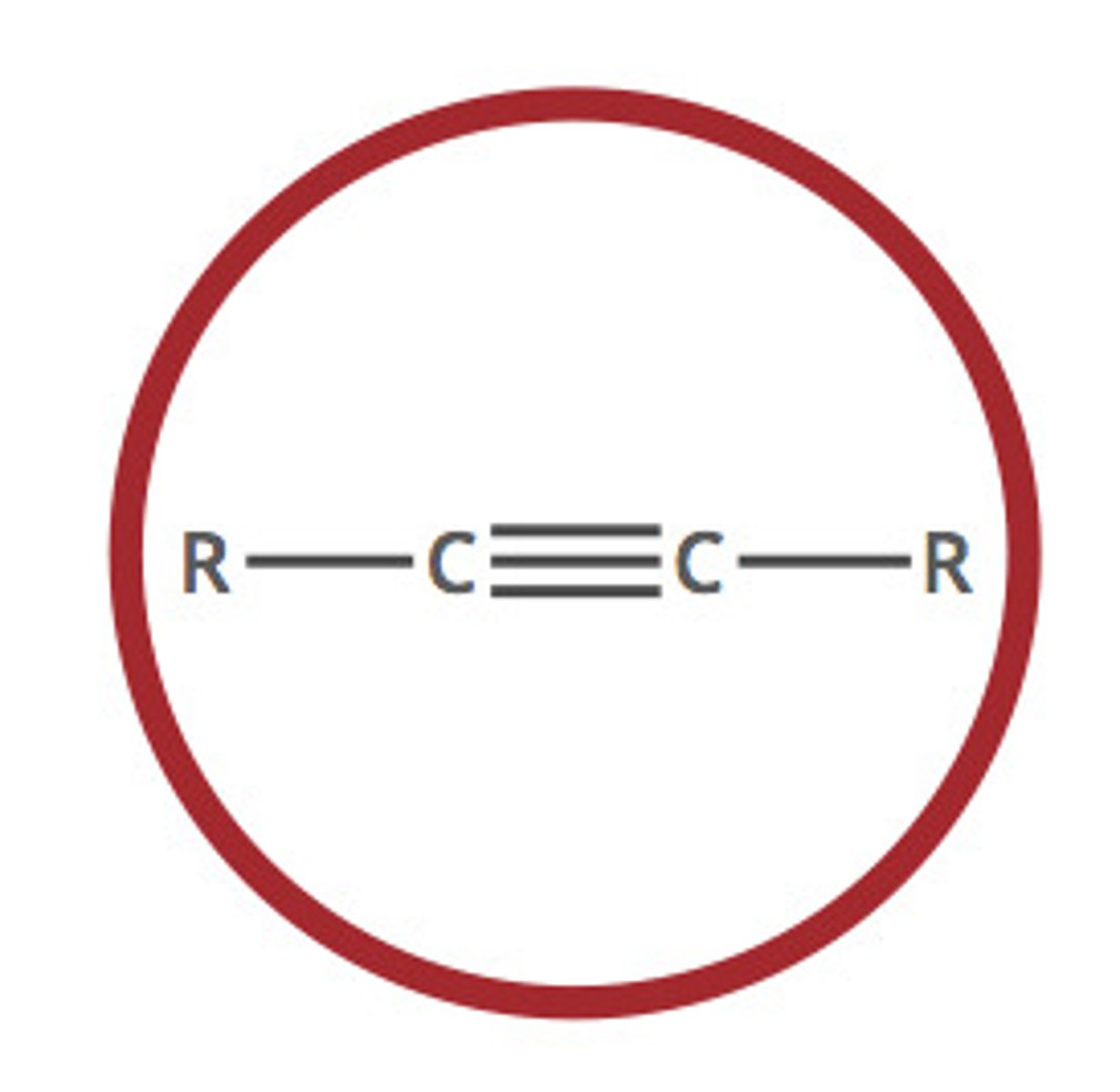
alkyne (-yne)
a hydrocarbon that contains one or more triple bonds
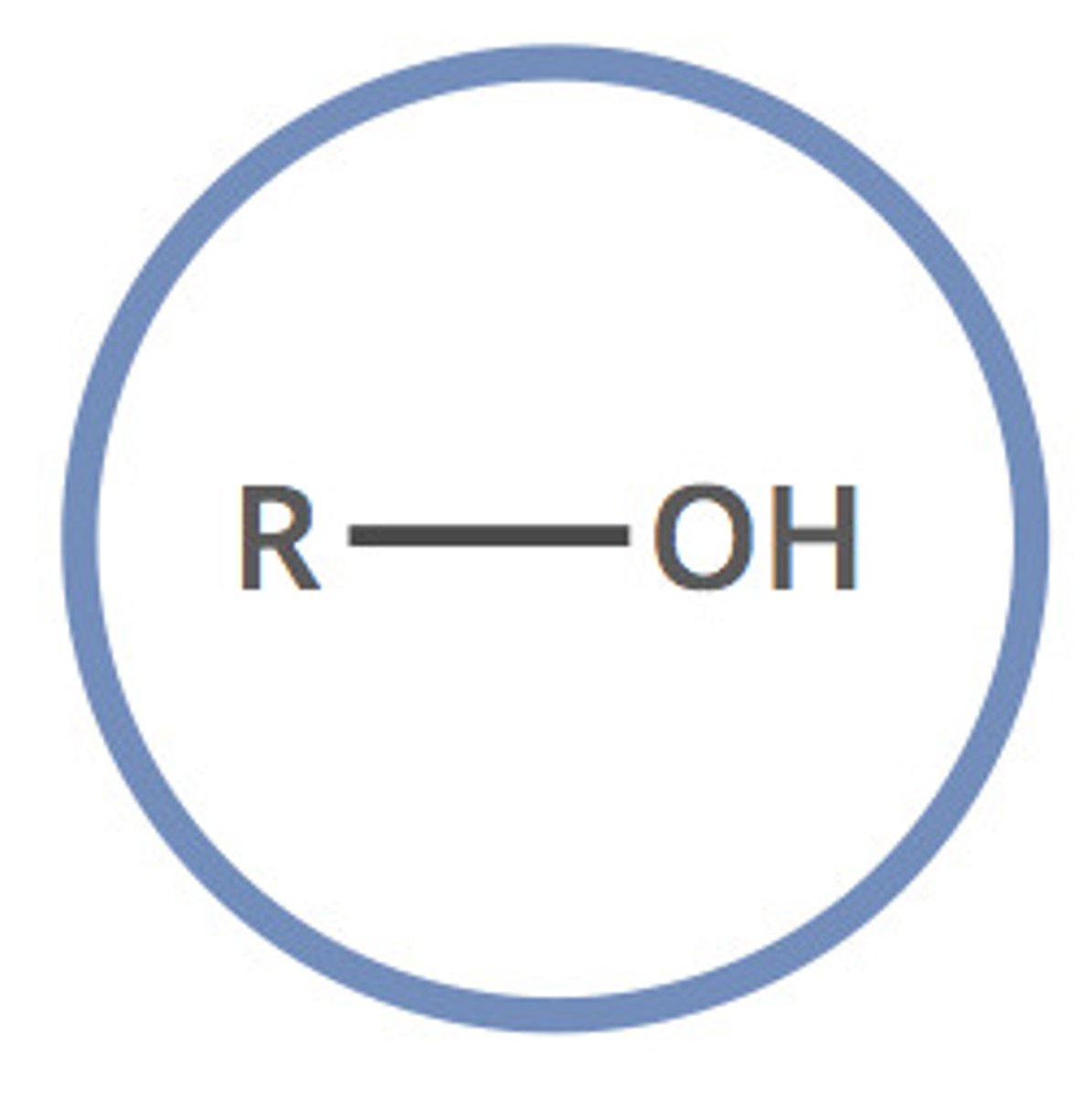
alcohol (-ol)
simple oxygen heteroatomic with a hydroxyl group and one alkyl group
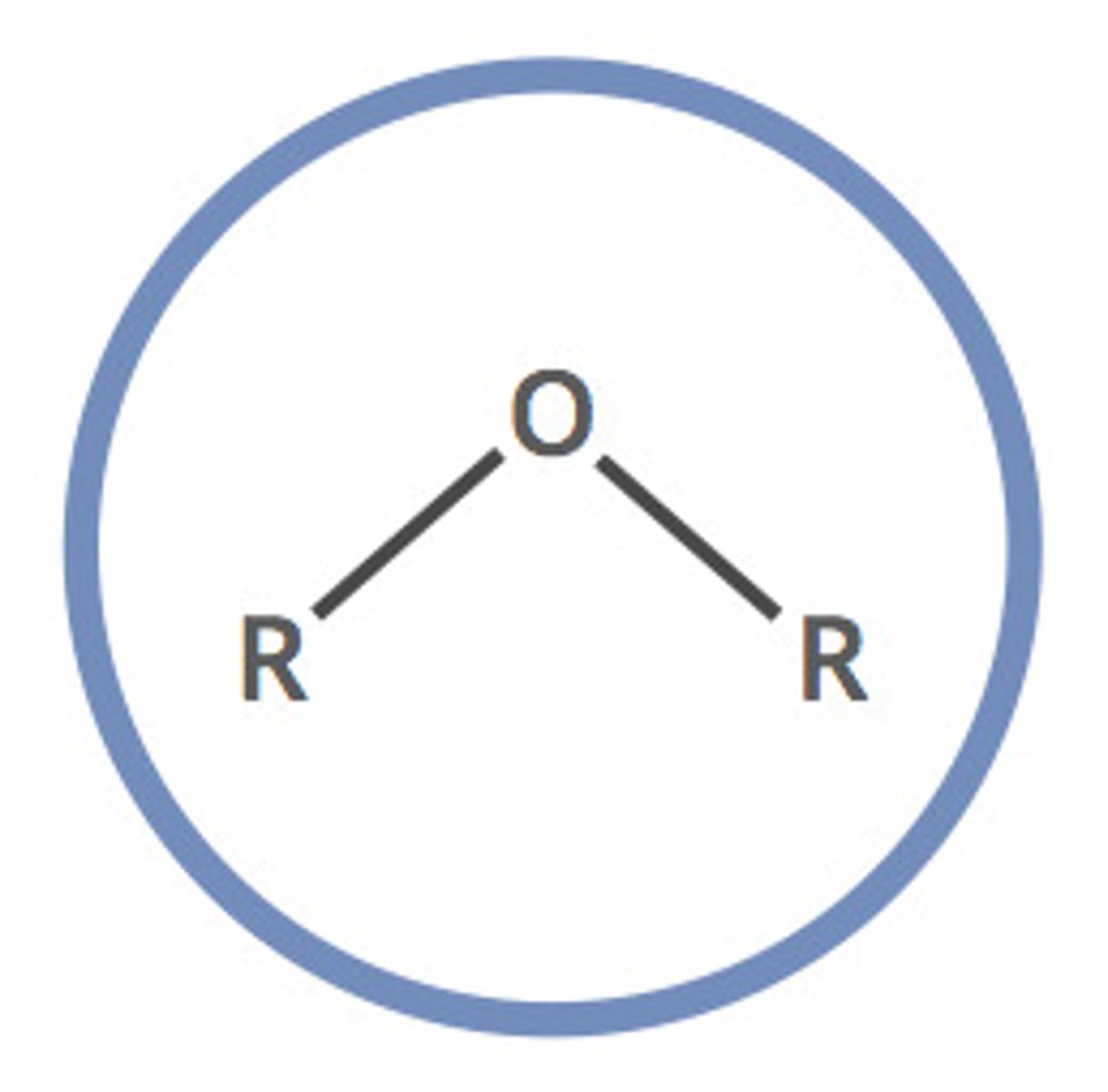
ether (-oxy -ane)
simple oxygen heteroatomic with two alkyl groups
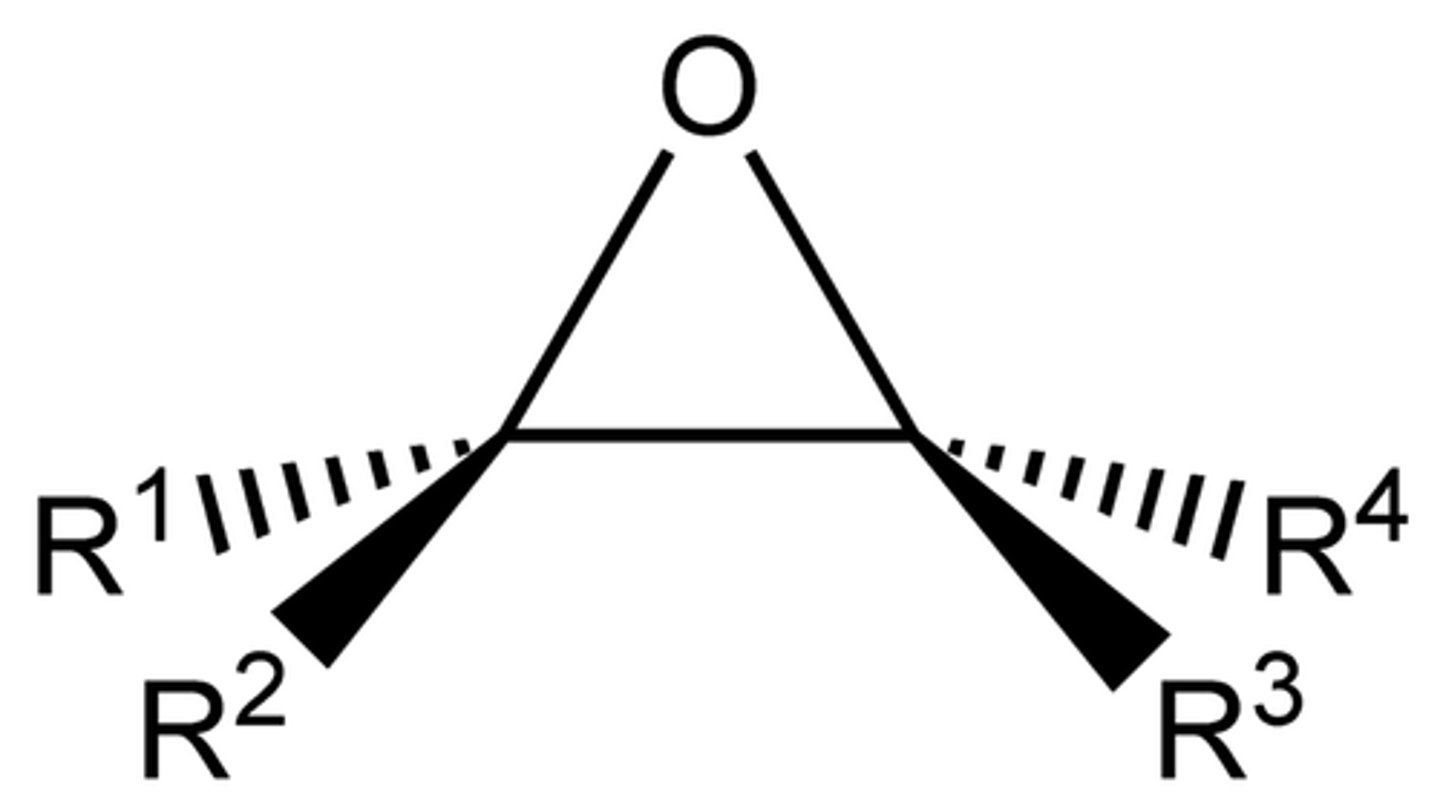
epoxide (-ene oxide)
an ether in which the oxygen is incorporated into a three-membered ring
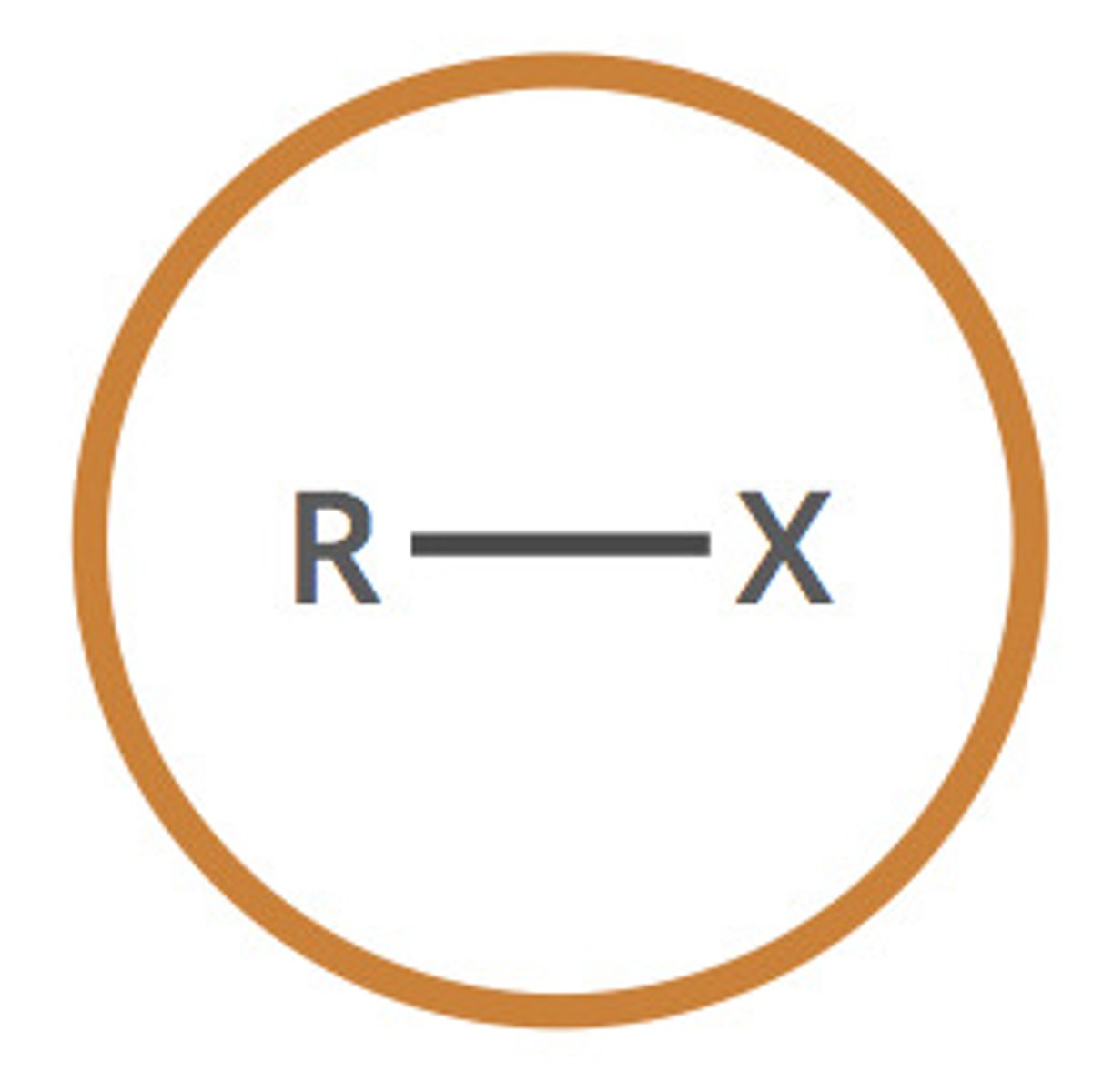
haloalkane (halo-)
alkane containing one or more halogens
aldehyde (-al)
organic molecule with a carbonyl group located at the end of the carbon skeleton
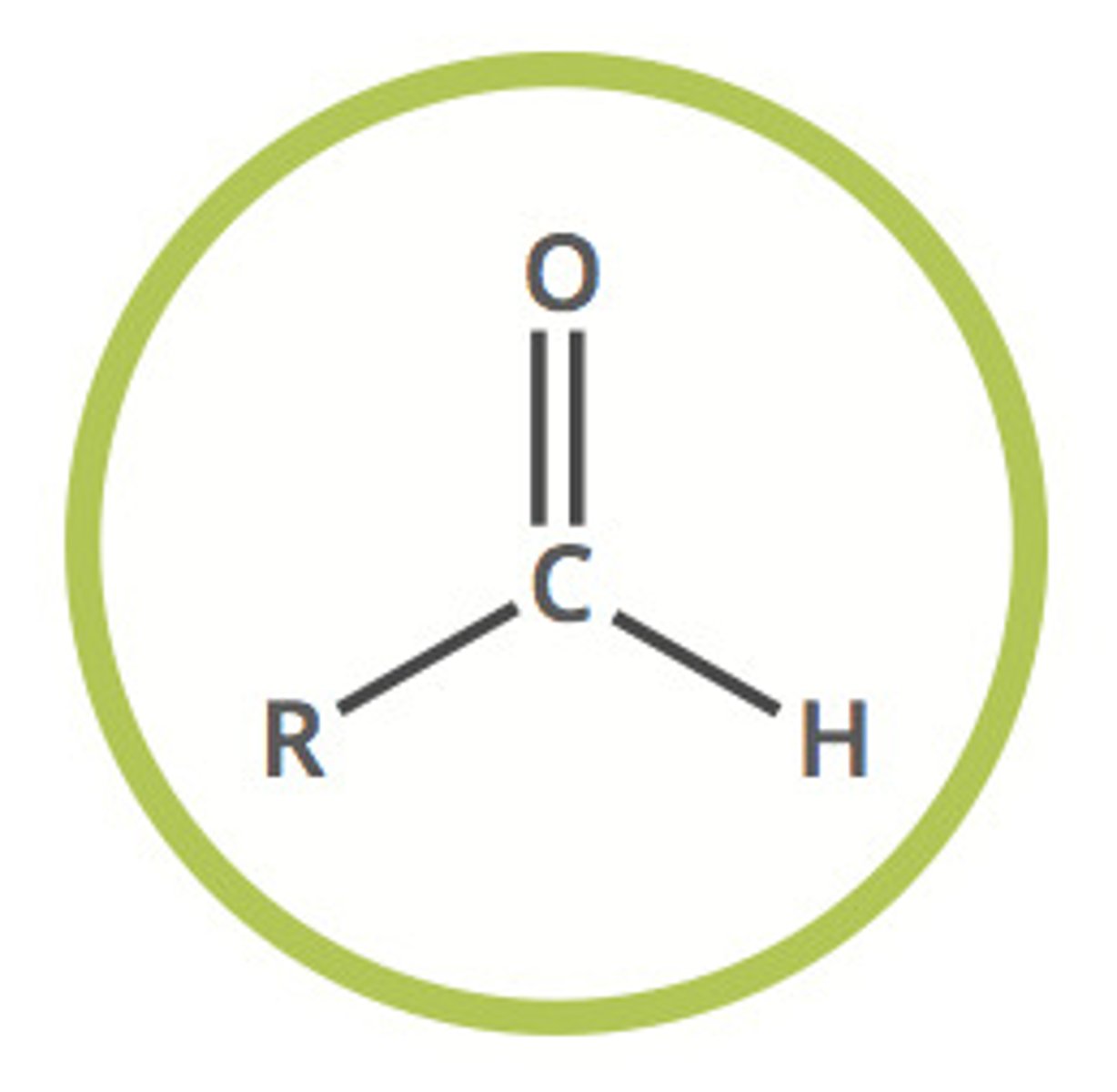
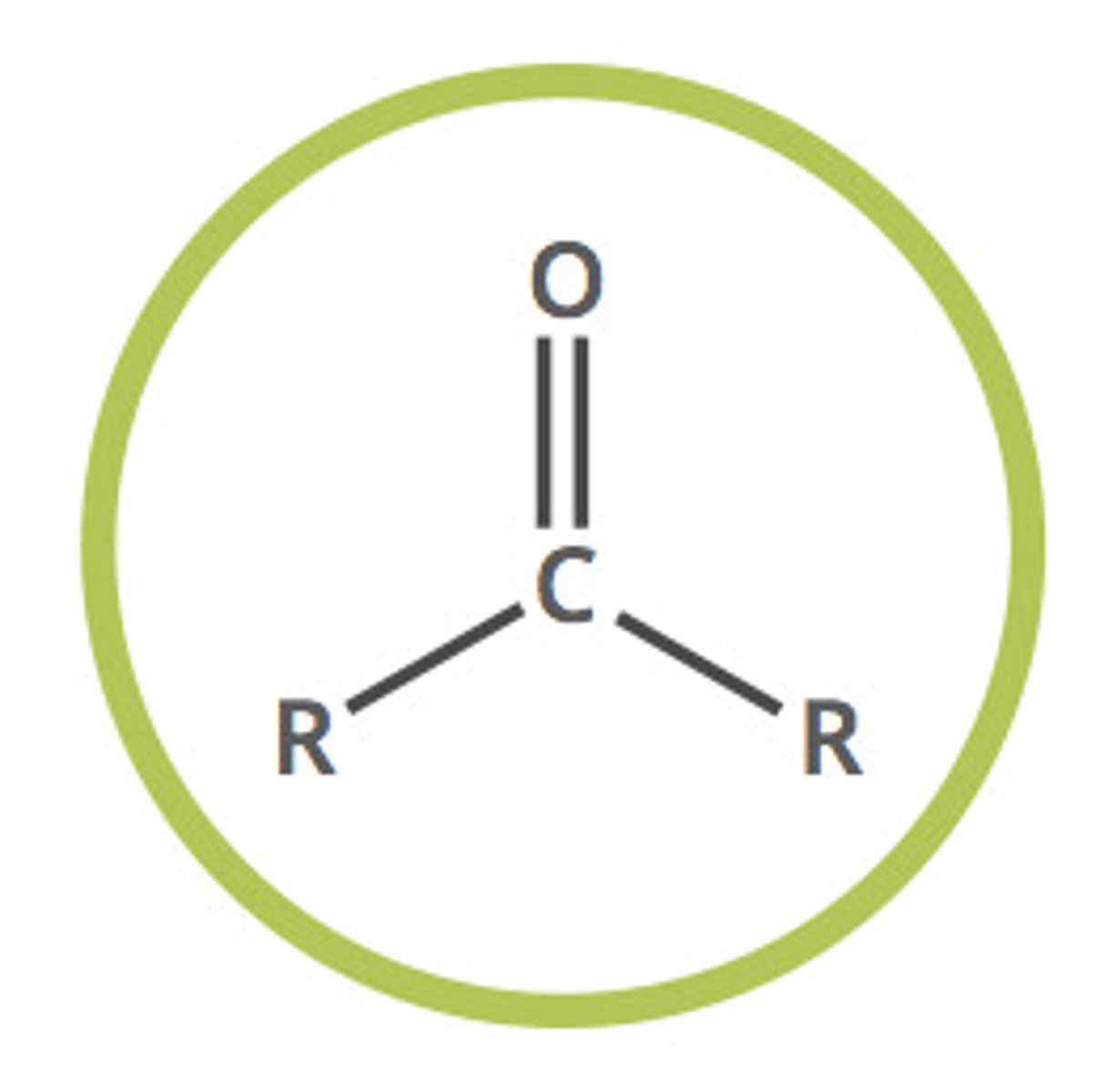
ketone (-one)
carbonyl compound with two alkyl groups
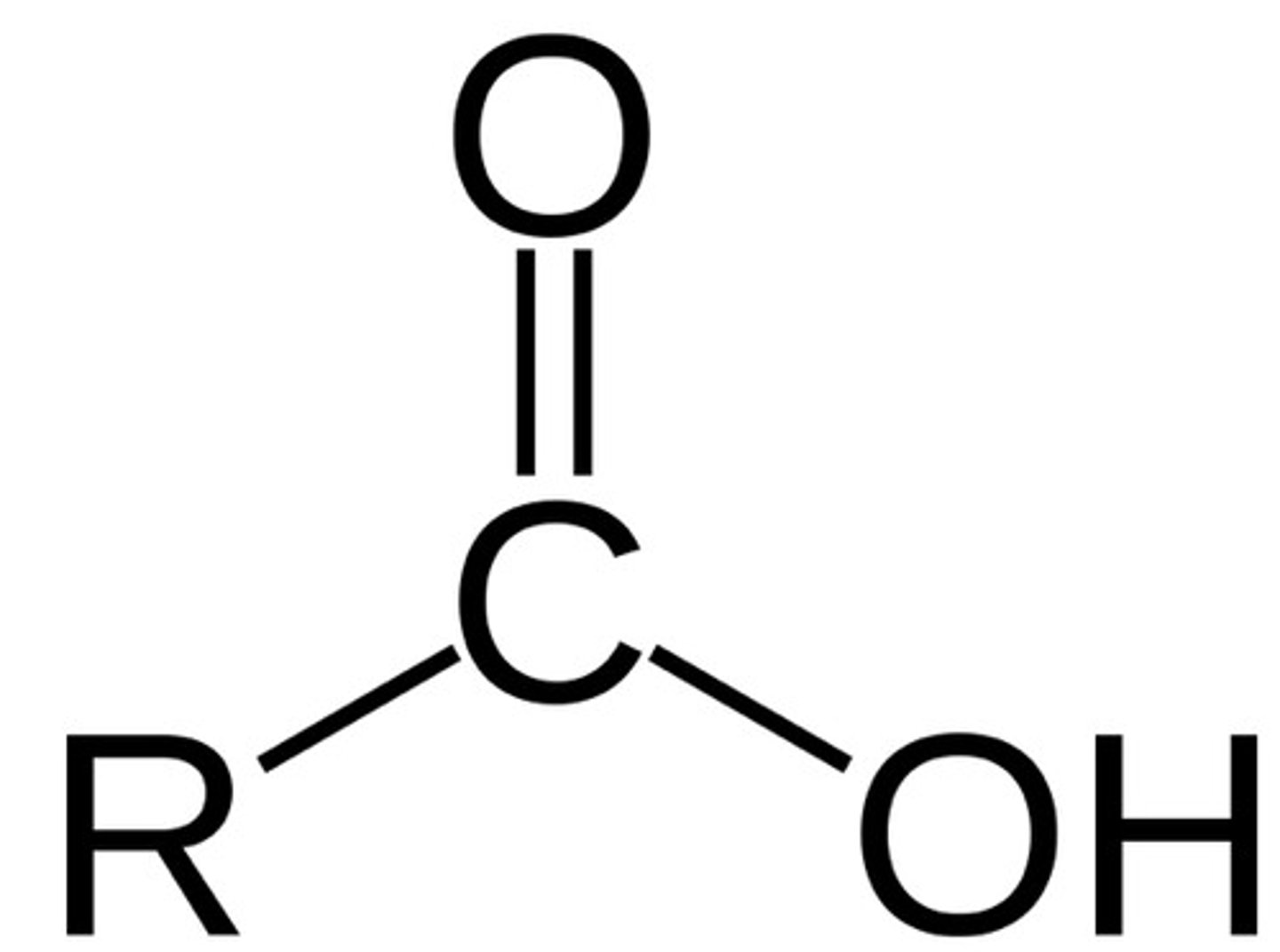
carboxylic acid (-oic acid)
carboxyl group attached to alkyl group
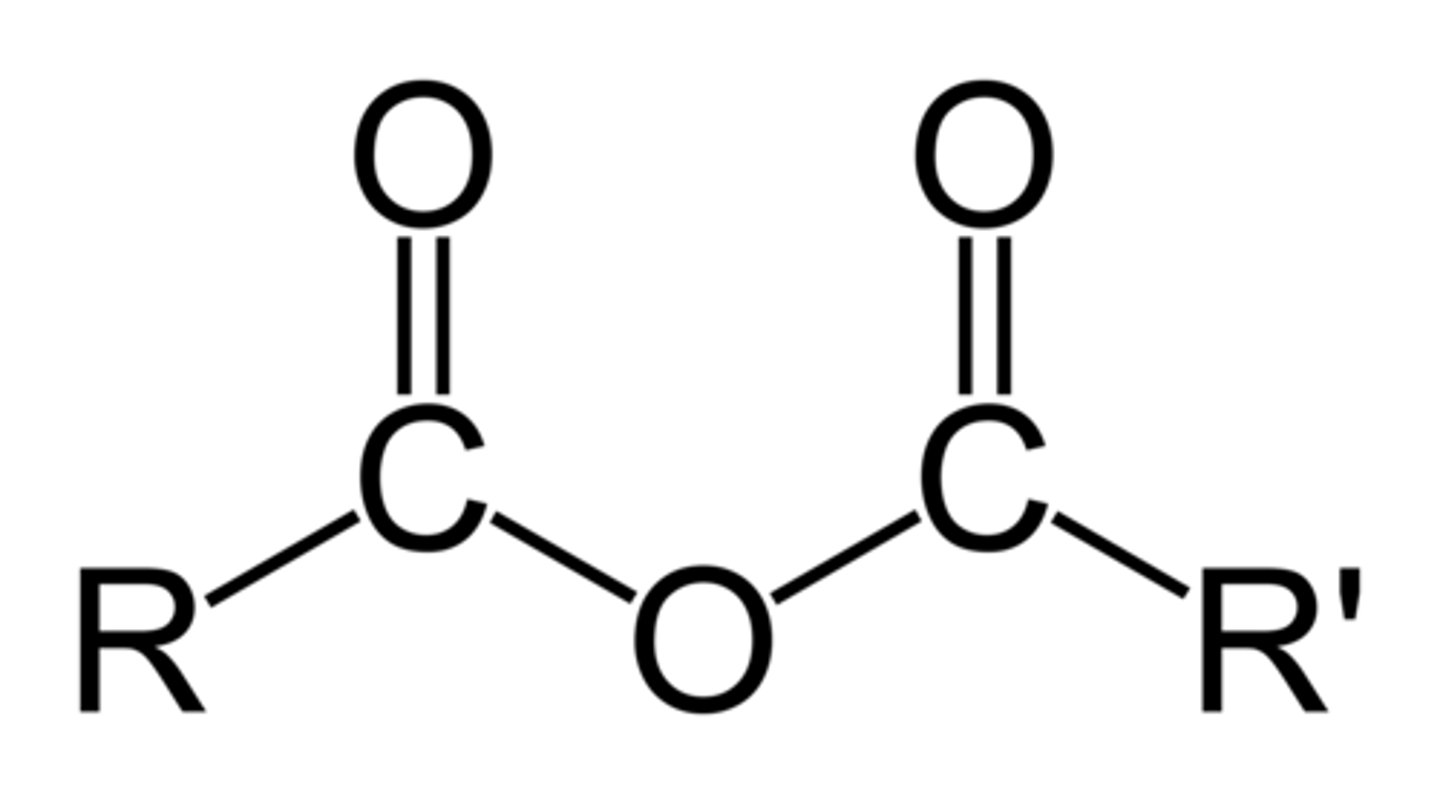
acid anhydride (-oic anhydride)
two acyl groups bonded to the same oxygen
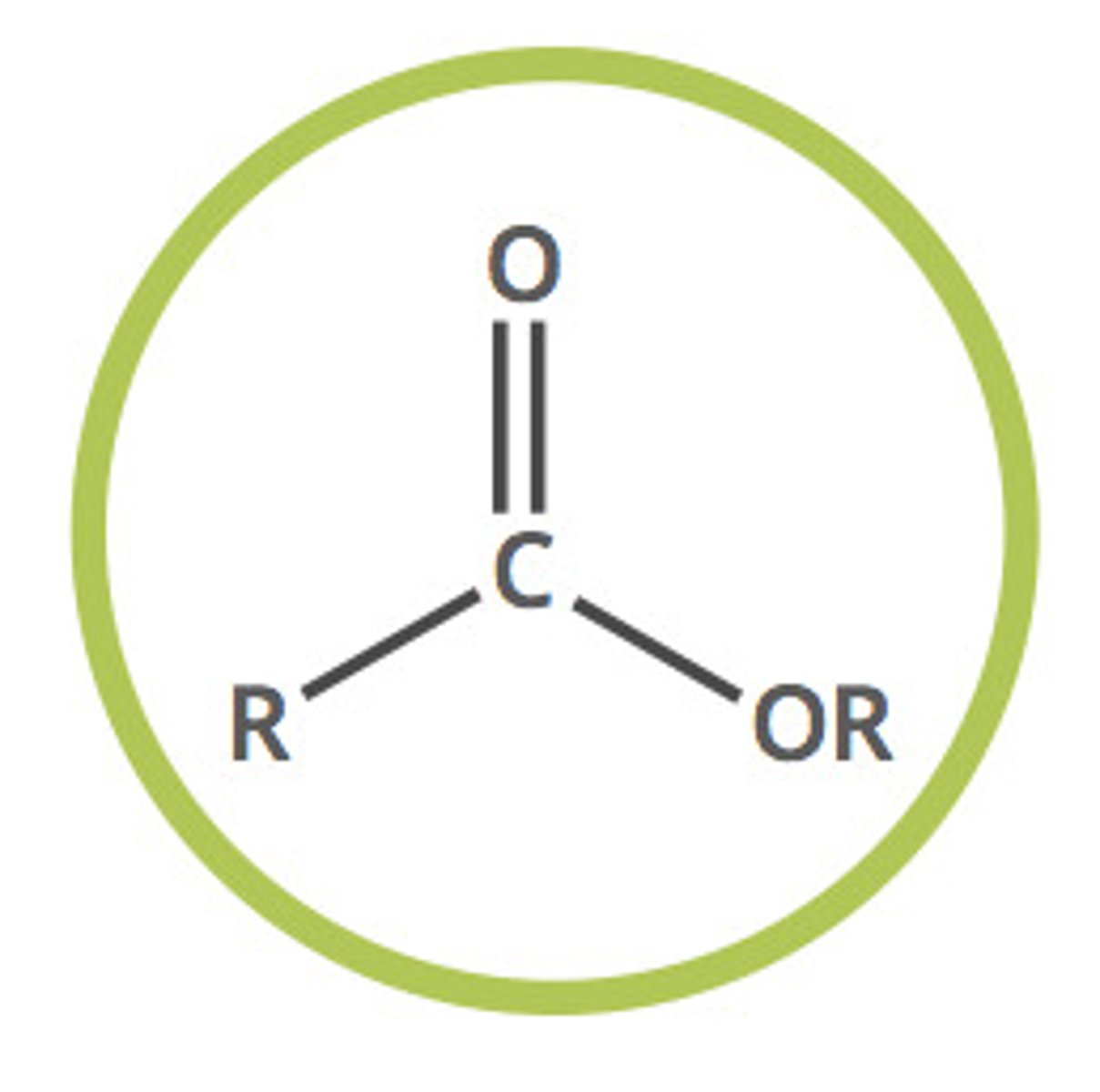
ester (-yl -oate)
compound derived from an acid in which at least one -OH hydroxyl group is replaced by an -O- alkyl group
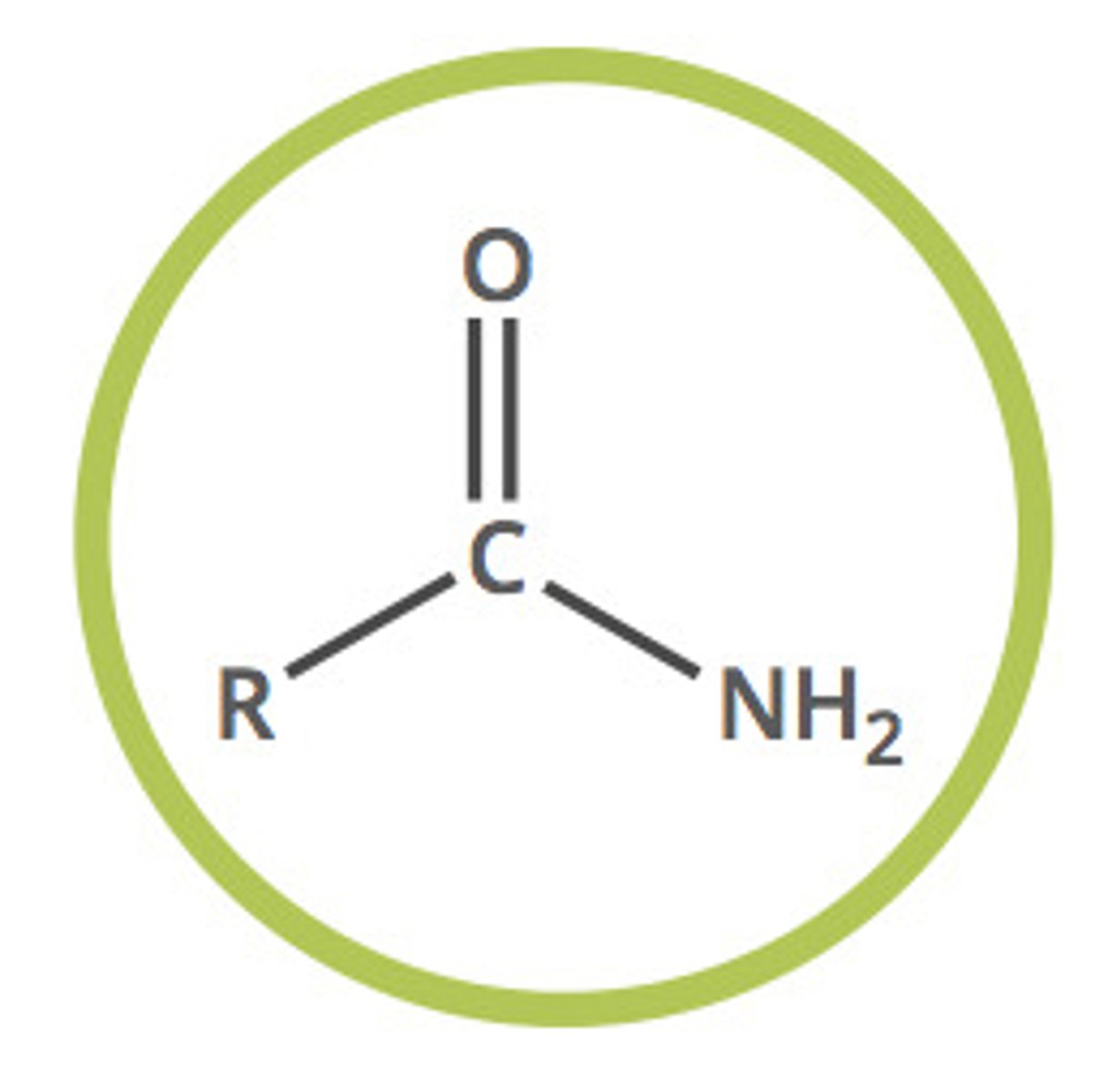
amide (-amide)
carbonyl carbon atom linked by a single bond to a nitrogen atom and either a hydrogen or a carbon atom
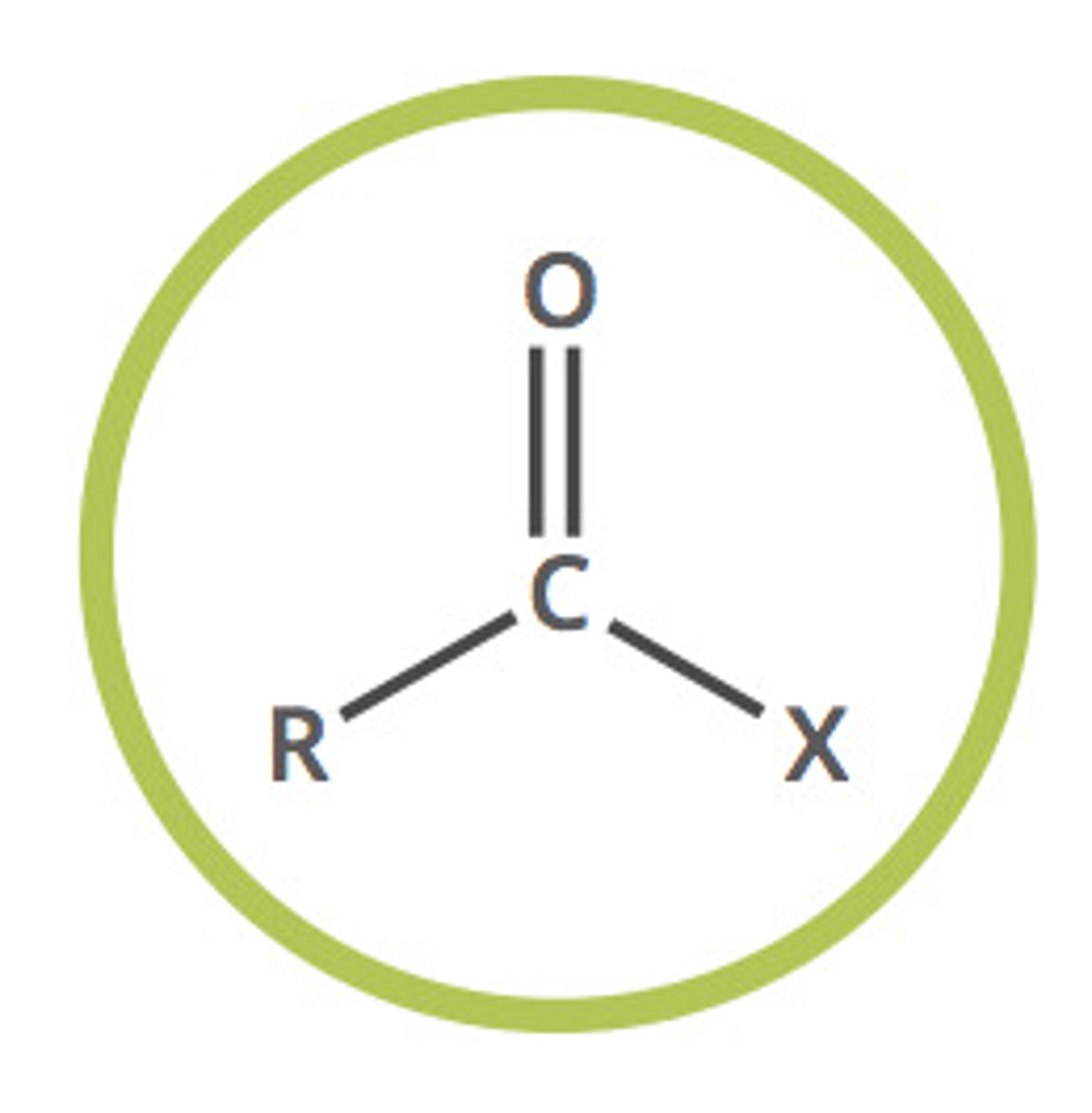
acyl halide (-oyl halide)
ketone with a side chain replaced by a halide group
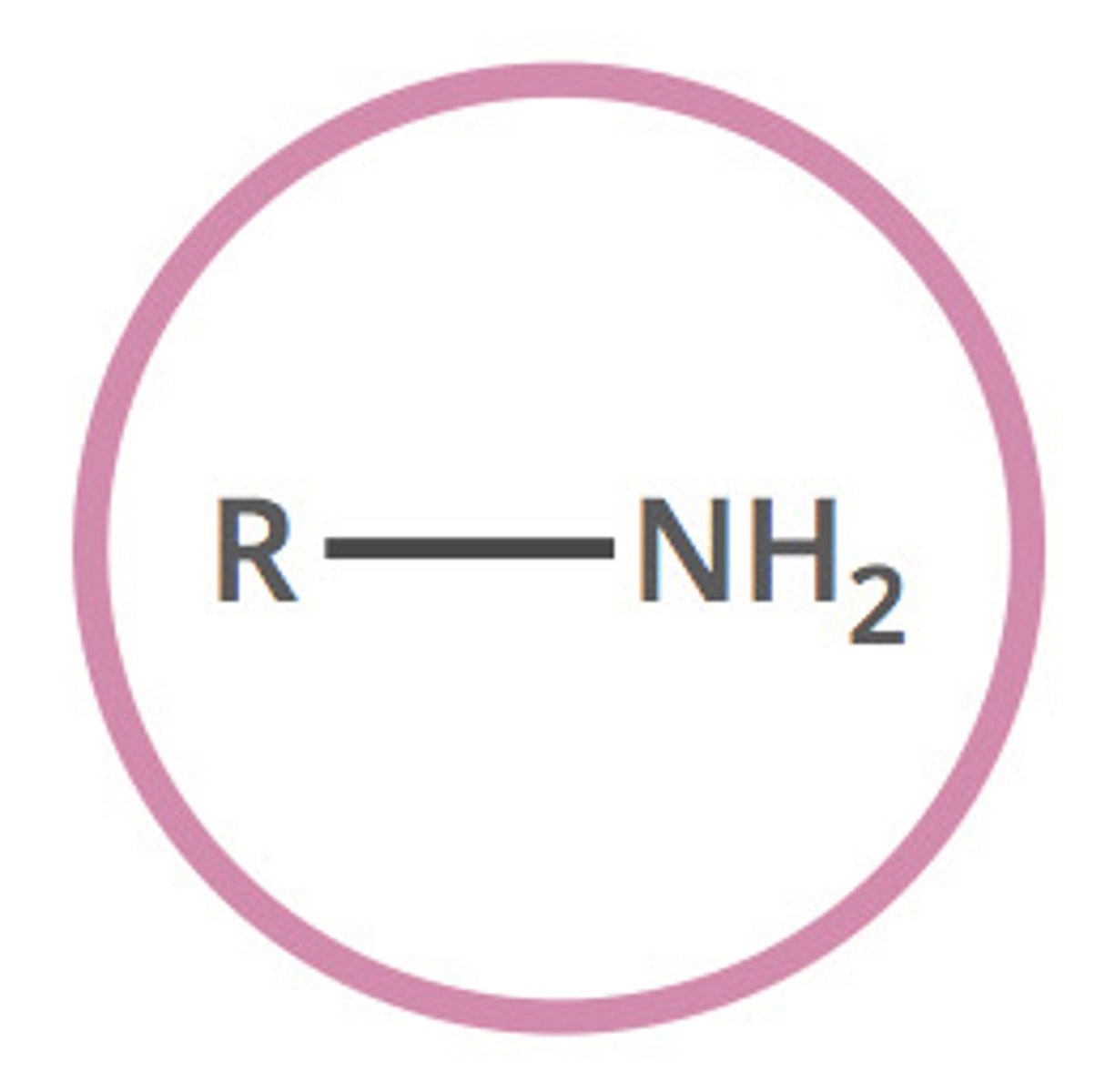
amine (-amine)
contains a basic nitrogen atom with a lone pair
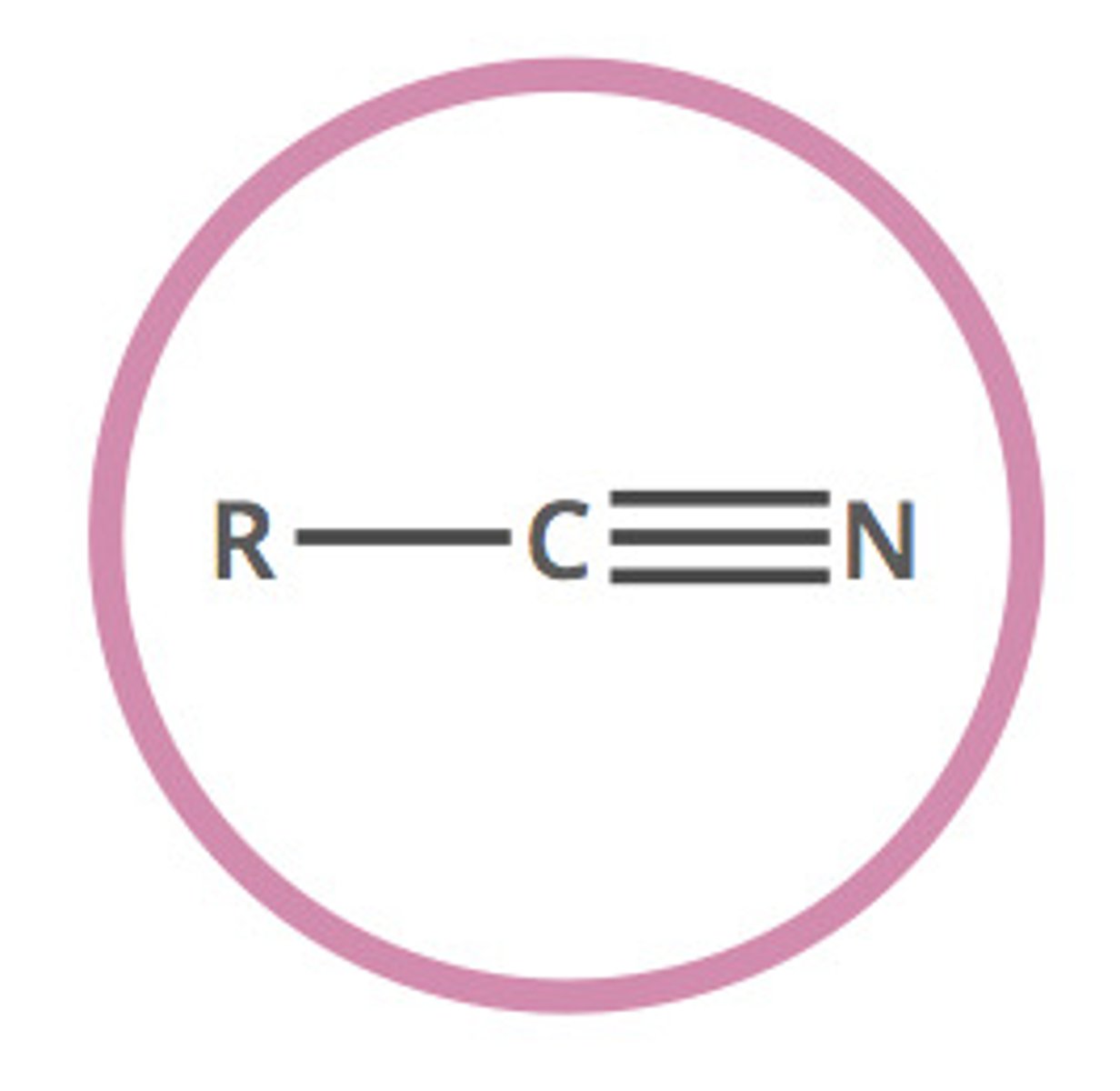
nitrile (-nitrile)
any organic compound that has a −C≡N functional group
imine (-imine)
compound with a N atom double-bonded to a carbon atom

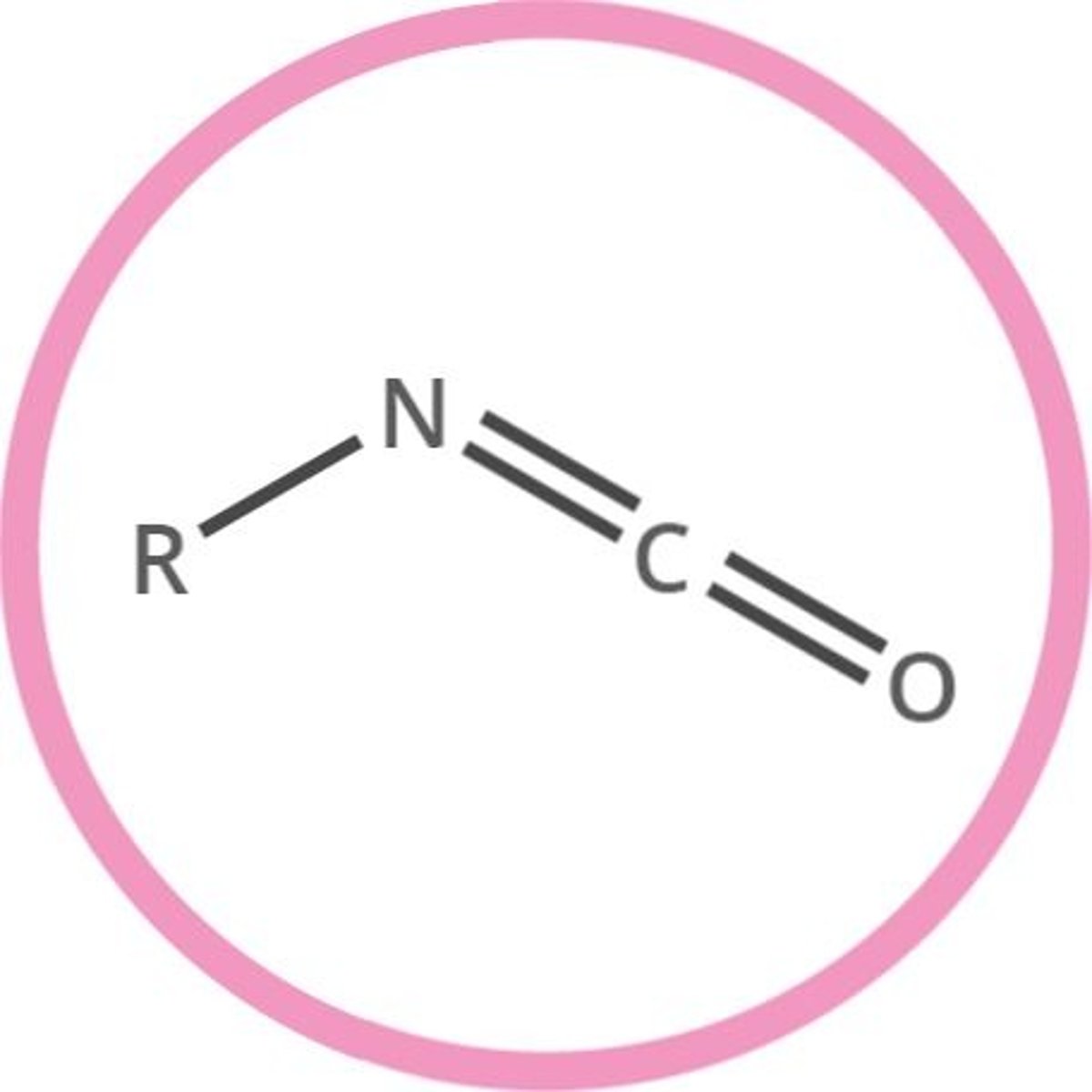
isocyanate (-yl isocyanate)
compound of formula R-N=C=O
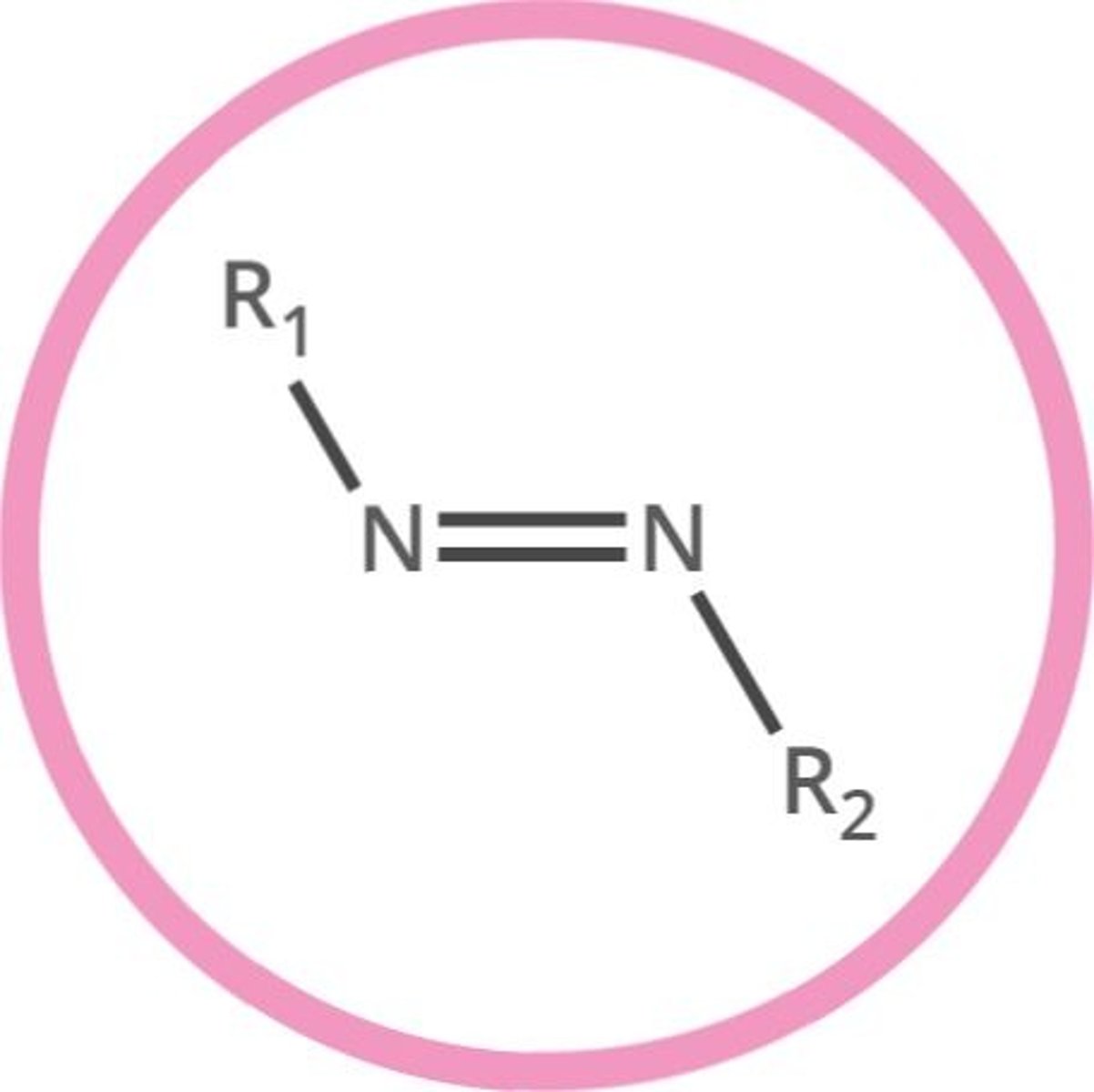
azo compound (azo-)
compound with formula R-N=N-R', where R and R' can be either aryl (aromatic) or alkyl (aliphatic) functional groups
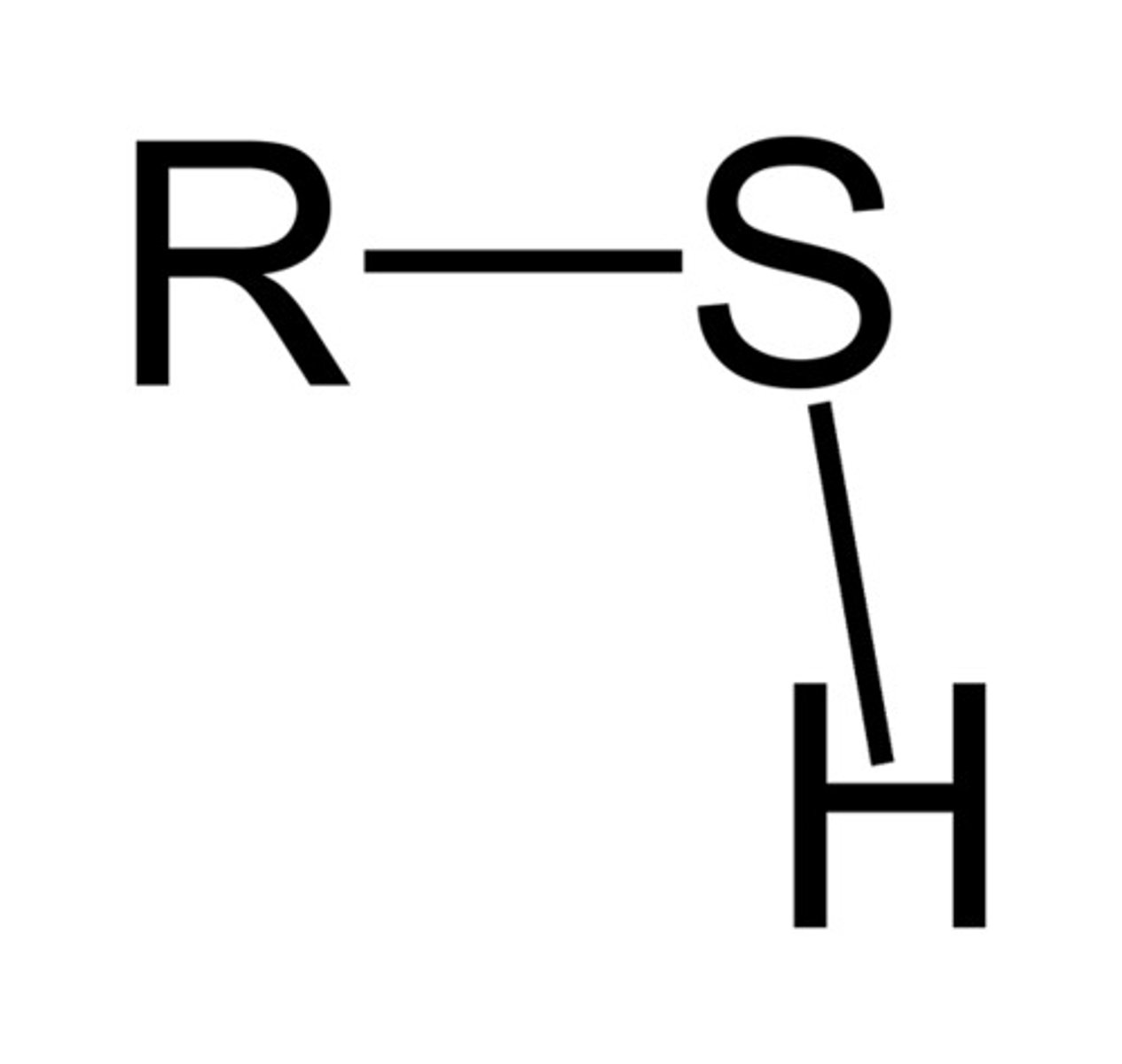
thiol (-thiol)
contains a carbon bonded to S-H
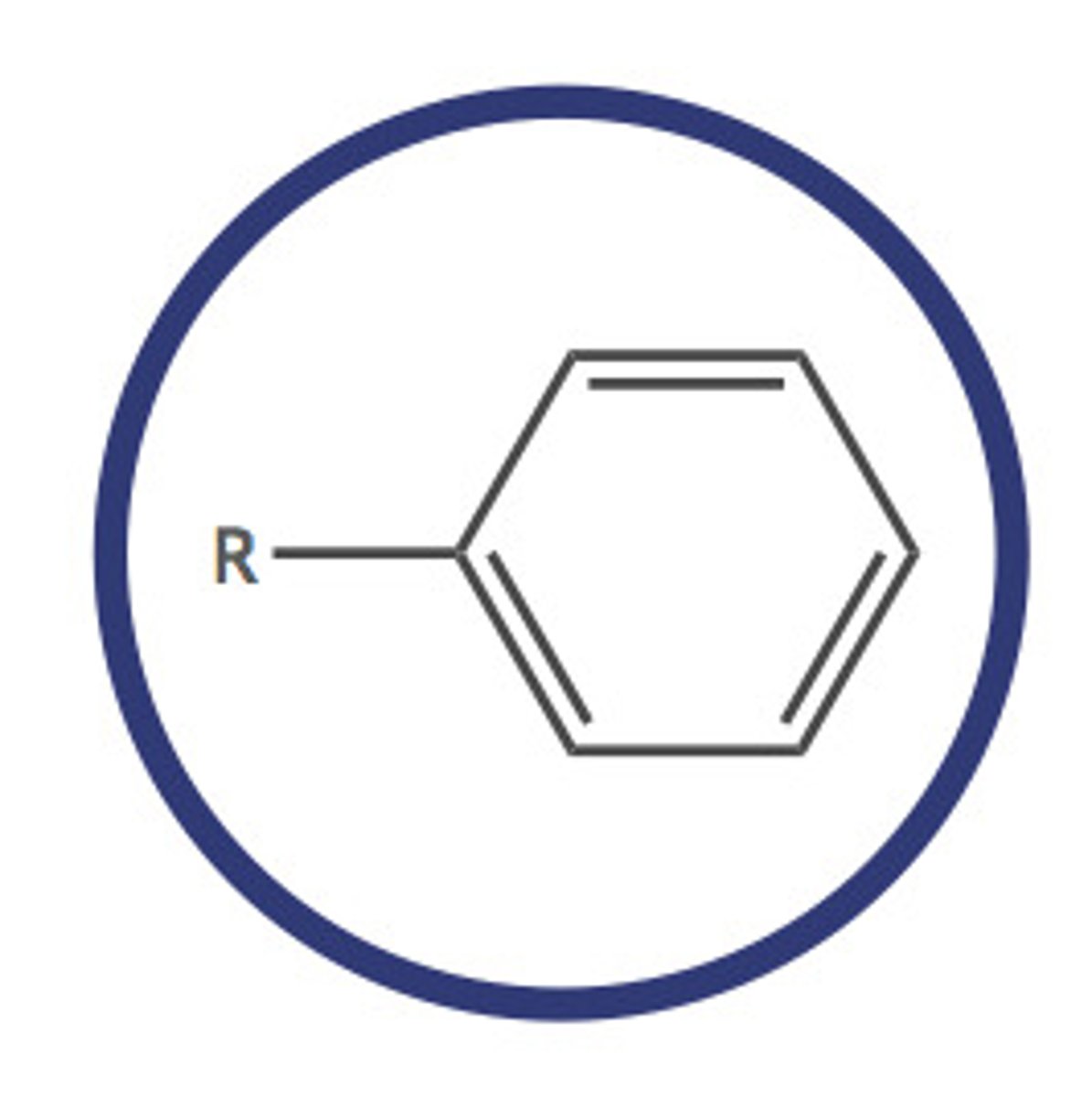
arene (-yl benzene)
hydrocarbon with alternating double and single bonds between carbon atoms forming rings