Science 8th Grade - The Periodic Table, Atoms, and Mendeleev
5.0(1)
5.0(1)
Card Sorting
1/29
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
1
New cards
Protons, neutrons, electrons, an electron cloud, and the nucleus.
What does the basic structure of an atom contain?
2
New cards
Protons, neutrons, and electrons.
What are the subatomic particles?
3
New cards
What is the mass of a proton?1 AMU
What is the mass of a proton?
4
New cards
1 AMU
What is the mass of a neutron?
5
New cards
1/2000 AMU
What is the mass of an electron?
6
New cards
Positive (+)
What is the charge of a proton?
7
New cards
None (0)
What is the charge of a neutron?
8
New cards
Negative (-)
What is the charge of an electron?
9
New cards
Protons and neutrons.
Which two subatomic particles are found in the nucleus?
10
New cards
Atoms of the same element that have different numbers of neutrons.
Isotopes
11
New cards
They have different amounts of neutrons.
What is the difference between each isotope?
12
New cards
Atomic number = protons = electrons.
What does APE stand for?
13
New cards
Mass number - atomic number = neutrons.
What does MAN stand for?
14
New cards
The protons and electrons cancel out each other.
Why is a stable atom's overall charge neutral even though it is made of charged particles?
15
New cards
The original increases by atomic mass, but it now increases by atomic number.
How is the modern periodic table different from Mendeleev's original periodic table?
16
New cards
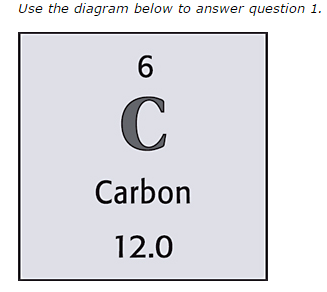
6
What is the atomic number of this element?
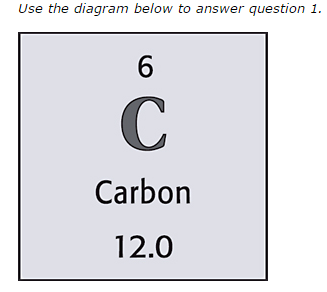
17
New cards
C
What is the symbol of this element?
18
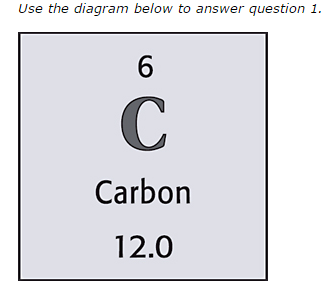
New cards
Carbon
What is the element?
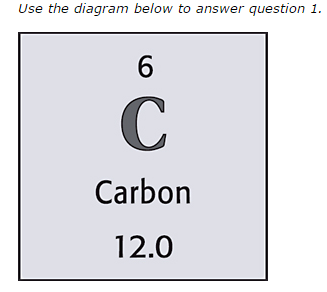
19
New cards
12
What is the atomic mass of the element?
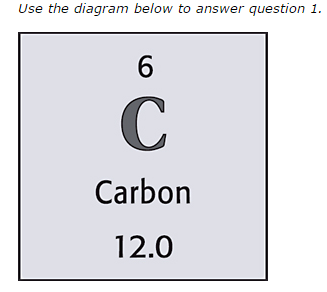
20
New cards
6
How many protons are there in this element?
21
New cards
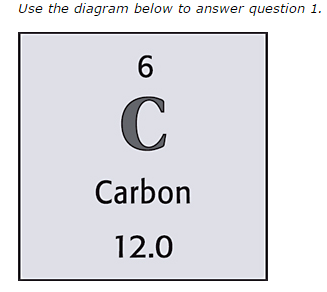
6
How many electrons are there in this element?
22
New cards
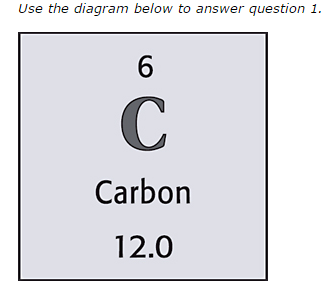
6
How many neutrons are there?
23
New cards
Are commonly referred to as 'rare earth metals'. They are placed off the table with Actinides so there's enough space and the periodic table is easier to read. They are often used for magnets, batteries, and hybrid cars.
Lanthanides
24
New cards
They are placed off the table with Lanthanides so there's enough space and the periodic table is easier to read. All of them are radioactive.
Actinides
25
New cards
These elements are not stable, decay radioactively into another element, and are not found in nature. These elements are made in a laboratory when nuclear particles are forced to crash into one another.
Transuranium Elements
26
New cards
These metals are located in the very left of the periodic table and are vert radioactive. These metals are also very shiny and soft.
Alkali Metals
27
New cards
These metals are very reactive and are hard and dense.
Alkaline Earth Metals
28
New cards
These metals take up most of the middle of the periodic table. They can also conduct electricity easily. They're hard, shiny, and have high melting points.
Transition Metals
29
New cards
These nonmetals are the most reactive of nonmetals.
Halogens
30
New cards
These elements are non-reactive.
Noble Gases