physics term 1
5.0(1)
5.0(1)
Card Sorting
1/51
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
52 Terms
1
New cards
atomic model description
An atom has a small, positively-charged nucleus surrounded by orbiting negatively-charged electrons.
\
\
2
New cards
density
mass kg / volume m³
3
New cards
upthrust
The force that keeps the object afloat
4
New cards
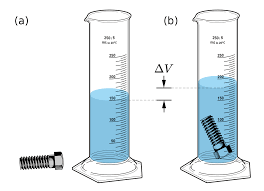
The Law of Displacement
The idea that an object completely submerged in a fluid (like water) will replace an amount of fluid equal to its own volume.
5
New cards
gases
.the least dense state of matter.
.The particles are free to move with negligible (tiny) forces between particles.
.The particles are free to move with negligible (tiny) forces between particles.
6
New cards
liquids
.This state is less dense than solids but more dense than gases.
.The particles in liquids can move around each other.
.The particles in liquids can move around each other.
7
New cards
solids
.They are the most dense state of matter.
.The particles are packed tightly together.
.The particles are packed tightly together.
8
New cards
calculating density of irregular shaped solids
* we can use the Law of Displacement to estimate its volume and a scale the find the mass.
* This is done using a displacement can, or a graduated measuring cylinder.
* The volume of displaced water is measured, and this is the volume of the object.
* This is done using a displacement can, or a graduated measuring cylinder.
* The volume of displaced water is measured, and this is the volume of the object.
9
New cards
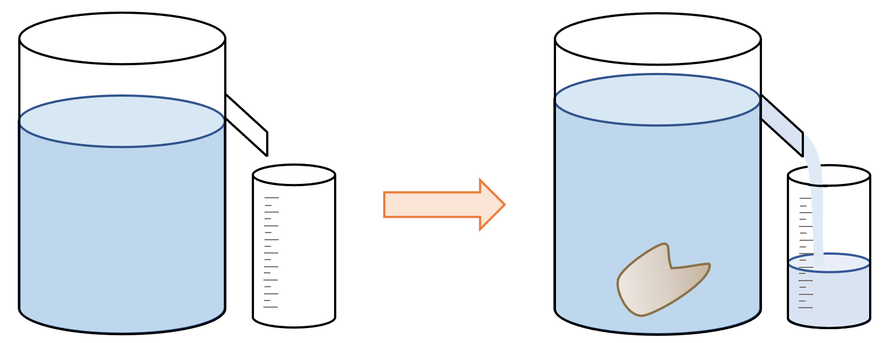
Displacement/ Eureka can
A can used to determine the volume of a solid.
10
New cards
calculating density of a liquid
* place a measuring cylinder on a scale and set the reading to zero.
* Pour the liquid into the cylinder and write down its mass and volume. Then calculate the density.
* Pour the liquid into the cylinder and write down its mass and volume. Then calculate the density.
11
New cards
liquids and solids
States of matter that cannot be compressed since their particles are already touching
12
New cards
gas and liquid
states of matter in which the particles are arranged in a disordered pattern.
13
New cards
gas
a state of matter that fills its container because it has no fixed volume and its particles constantly move and spreads out.
14
New cards
liquid
a state of matter that takes the shape of its container
15
New cards
solid
a state of matter that has a fixed shape and arranged in an ordered pattern. It has a fixed shape because
16
New cards
conservation of mass
When a substance changes state, its mass is conserved (stays the same).
17
New cards
change of state is a physical process
when a substance changes state, it is reversible meaning it isn’t a chemical change
18
New cards
melting point
fusion point
19
New cards
kinetic theory of matter
The idea that all 3 states of matter are made of particles that are constantly moving
20
New cards
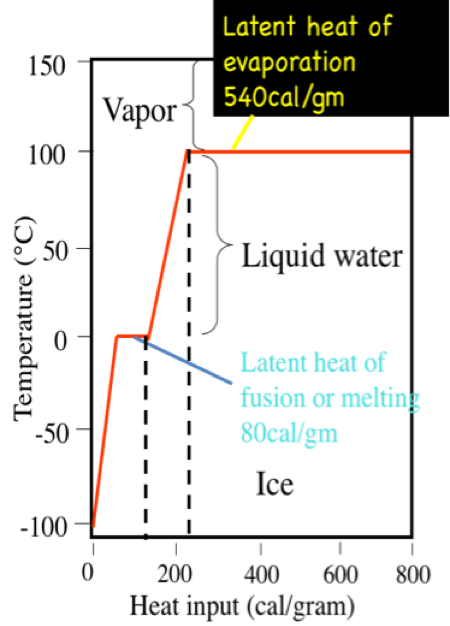
latent heat
the energy that is transferred to a substance without the substance's temperature changing. This happens when a substance is changing state.
21
New cards
specific latent heat
the latent heat per 1kg of mass. It is a way to standardise across objects that have different masses
22
New cards
what happens to latent heat
When a substance changes state, the temperature stops increasing because this energy is used to create or weaken bonds, rather than transfer kinetic energy to a substance’s particles.
23
New cards
heat
The amount of kinetic something has. It is measure in joules (J)
24
New cards
temperature
the measure of the average internal energy of all the particles in a substance. It is measured in degrees Celsius (⁰C) or kelvin (K)
25
New cards
temperature
The measure of how hot or cold something is
26
New cards
latent heat of fusion
When a solid becomes a liquid or a liquid becomes a solid, this hidden energy is called xxx
27
New cards
latent heat of vaporisation
When a gas becomes a liquid, or a liquid becomes a gas, this hidden energy is called xxx
28
New cards
calculating energy change for change of state
energy (j) =mass (kg) × latent heat (j/kg)
29
New cards
detecting latent heat
using a joulemeter and measuring energy supplied to change state
30
New cards
potential energy
energy stored in an object
31
New cards
internal energy
the total amount of energy in chemical and kinetic stores
32
New cards
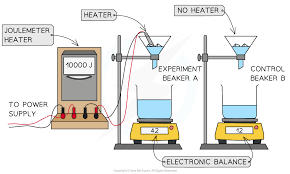
specific latent heat of fusion experiment
* To determine water’s specific latent heat of fusion we use the equation: \n specific latent heat = energy change ÷ mass.
* Gently heat ice in a funnel until it melts. Then measure the mass of the melted ice (water in the beaker).
* Measure the amount of energy supplied by the heater using a joulemeter (this gives us the energy change).
* Calculate the specific latent heat of fusion using our equation above.
* Gently heat ice in a funnel until it melts. Then measure the mass of the melted ice (water in the beaker).
* Measure the amount of energy supplied by the heater using a joulemeter (this gives us the energy change).
* Calculate the specific latent heat of fusion using our equation above.
33
New cards
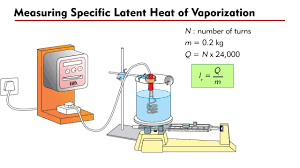
specific latent heat of vaporisation experiment
* To determine water’s specific latent heat of vapourisation we use the equation: \n specific latent heat = energy change ÷ change in mass.
* Measure the mass of water in a beaker.
* Boil some water and then measure the mass of the water again.
* Mass at the start - mass at the end = change in mass.
* Measure the amount of energy supplied by the heater using a joulemeter (this gives us the energy change).
* Calculate the latent heat of vapourisation using our equation above.
* Measure the mass of water in a beaker.
* Boil some water and then measure the mass of the water again.
* Mass at the start - mass at the end = change in mass.
* Measure the amount of energy supplied by the heater using a joulemeter (this gives us the energy change).
* Calculate the latent heat of vapourisation using our equation above.
34
New cards
joulemeter
a device that measures the energy supplied
35
New cards
Thermal heat capacity experiment
The specific heat capacity of a substance is the amount of energy needed to increase the temperature of 1 kg of that substance by 1 °C.
* The heater increases the internal energy of the body and we measure this using a joulemeter.
* Measure the temperature of the body (object) at the start and measure the maximum temperature of the body at the end.
* Specific heat capacity = change in internal energy / (mass (kg) x maximum temperature rise (°C) ).
* The heater increases the internal energy of the body and we measure this using a joulemeter.
* Measure the temperature of the body (object) at the start and measure the maximum temperature of the body at the end.
* Specific heat capacity = change in internal energy / (mass (kg) x maximum temperature rise (°C) ).
36
New cards
specific heat capacity equation
specific heat capacity (j/kg/⁰C) = change in internal energy (j) / (mass (kg) x maximum temperature rise (⁰C)
\
\
37
New cards
change in internal energy ( Δ*E*=*mc*Δ*θ* ) equation
change in internal energy (kj) =mass (kg) × specific heat capacity (j/kg/°C)× temperature change (⁰C)
divide the answer by 1000 to turn it into kj
divide the answer by 1000 to turn it into kj
38
New cards
thermal heat capacity equation
thermal capacity=mass (kg) ×specific heat capacity (J/KG/°C)
39
New cards
Atmospheric Pressure
the force per unit of area created by the weight of the air (particles) in the atmosphere.
40
New cards
pressure equation
pressure (p)= force (N) / area (m²)
41
New cards
Liquid pressure
As you dive deeper into a swimming pool, there is more water (and weight) on top of you. This extra weight exerts a larger force (and higher pressure) on your body. The deeper down you swim, the more pressure you feel.
42
New cards
liquid pressure equation
Liquid pressure (p) = density (g/cm³) x gravitational field strength (N/kg) x depth (m)
43
New cards
depth underwater
Depth is equal to the height of the column of water above you.
44
New cards
baking bread
**When you put bread in the oven, the temperature of the bread rises to 200°C. The air particles in the bread have more kinetic energy and exert pressure on the bread from the inside. This creates air bubbles that expand, causing the bread to rise.**
45
New cards
force exerted by fluid
The force exerted on the surface in contact with the fluid particle will be at the normal to the surface (at right angles)
46
New cards
how snow shoes work
A snowshoe has a much wider surface area than a standard shoe. This means that for a person weighing 80kg, the pressure exerted (placed) on the snow is smaller if they wear snowshoes instead of trainers. This means that the person is less likely to sink into the snow.
47
New cards
Temperature of gases
As you heat a gas, you transfer more kinetic energy to the gas' particles.
48
New cards
pressure of gasses
* A gas exerts pressure on the walls of its container.
* There are lots of gas particles colliding with the container each second.
* When a gas particle collides with the wall of its container, its momentum changes and it bounces back off the wall.
* This exerts a force on both the particle and the wall.
* The pressure exerted on the wall is equal to the force (of the ball) per unit area (of the wall being hit).
* There are lots of gas particles colliding with the container each second.
* When a gas particle collides with the wall of its container, its momentum changes and it bounces back off the wall.
* This exerts a force on both the particle and the wall.
* The pressure exerted on the wall is equal to the force (of the ball) per unit area (of the wall being hit).
49
New cards
momentum equation
momentum (kg/m/s) =mass(*kg*)×velocity(m/s)
50
New cards
an impulse
a change in momentum
51
New cards
constant equation
constant = pressure (p) x volume (m³)
52
New cards
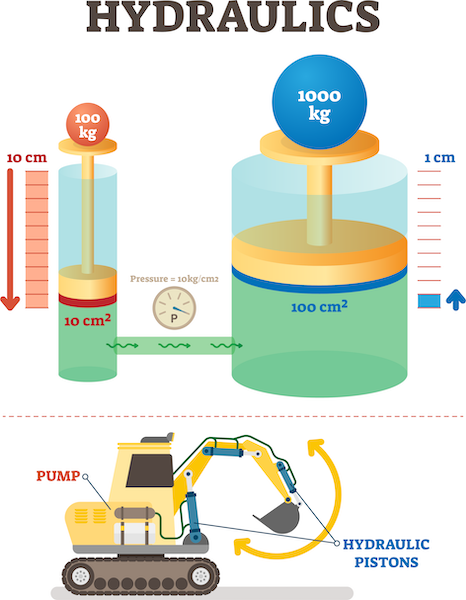
hydraulic systems
These systems use fluids to transfer forces from one place to another. The pressure is the same throughout the system.