Chapter 21 Biochemistry
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
53 Terms
Extracellular and intracellular proteins may be digested by …
lysosomal proteases
Other proteins to be degraded are first …
conjugated to the protein ubiquitin
The proteasome, a _____________, unfolds ____________ in an ___________ process and ____________ degrades them.
barrel-shaped complex; ubiquitinated proteins; ATP-dependent; proteolytically
The components of living cells are ______________. Proteins have lifetimes that range from as short as ____________ to ___________. In any case, cells continuously _____________ from and ______________. This seemingly wasteful process has three functions:
constantly turning over; a few minutes; weeks or more; synthesize proteins; degrade them to amino acids
1. to store nutrients in the form of proteins and to break them down in times of metabolic need, processes that are most significant in muscle tissue.
2. to eliminate abnormal proteins whose accumulation would be harmful to the cell.
3. to permit the regulation of cellular metabolism by eliminating superfluous enzymes and regulatory proteins.
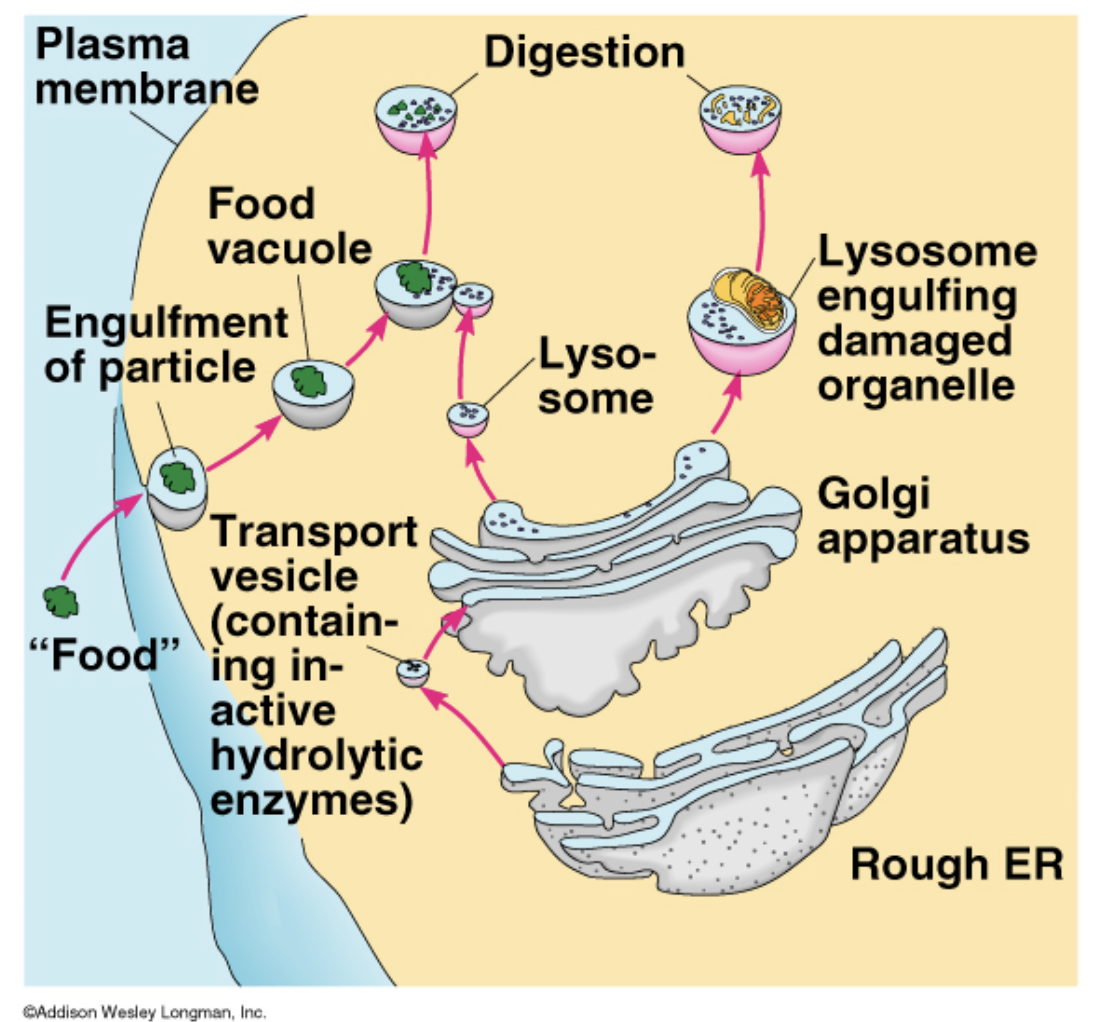
A lysosome is a _______________ found in ____________. They are structurally and chemically ___________ containing _____________, which are capable of _______________, including ______________. They are known to contain more than _____________ which are all active at an _____________. Thus they act as a _____________ by ______________, both from _____________ and ____________.
membrane-bound cell organelle; animal cells; spherical vesicles; hydrolitic enzymes; breaking down virtually all kinds of biomolecules; proteins, nucleic acids, carbohydrates, lipids, and cellular debris; 50 different; acidic environment of about pH 5; waste disposal system of the cell; digesting unwanted materials in the cytoplasm; outside of the cell; obsolete components inside the cell.
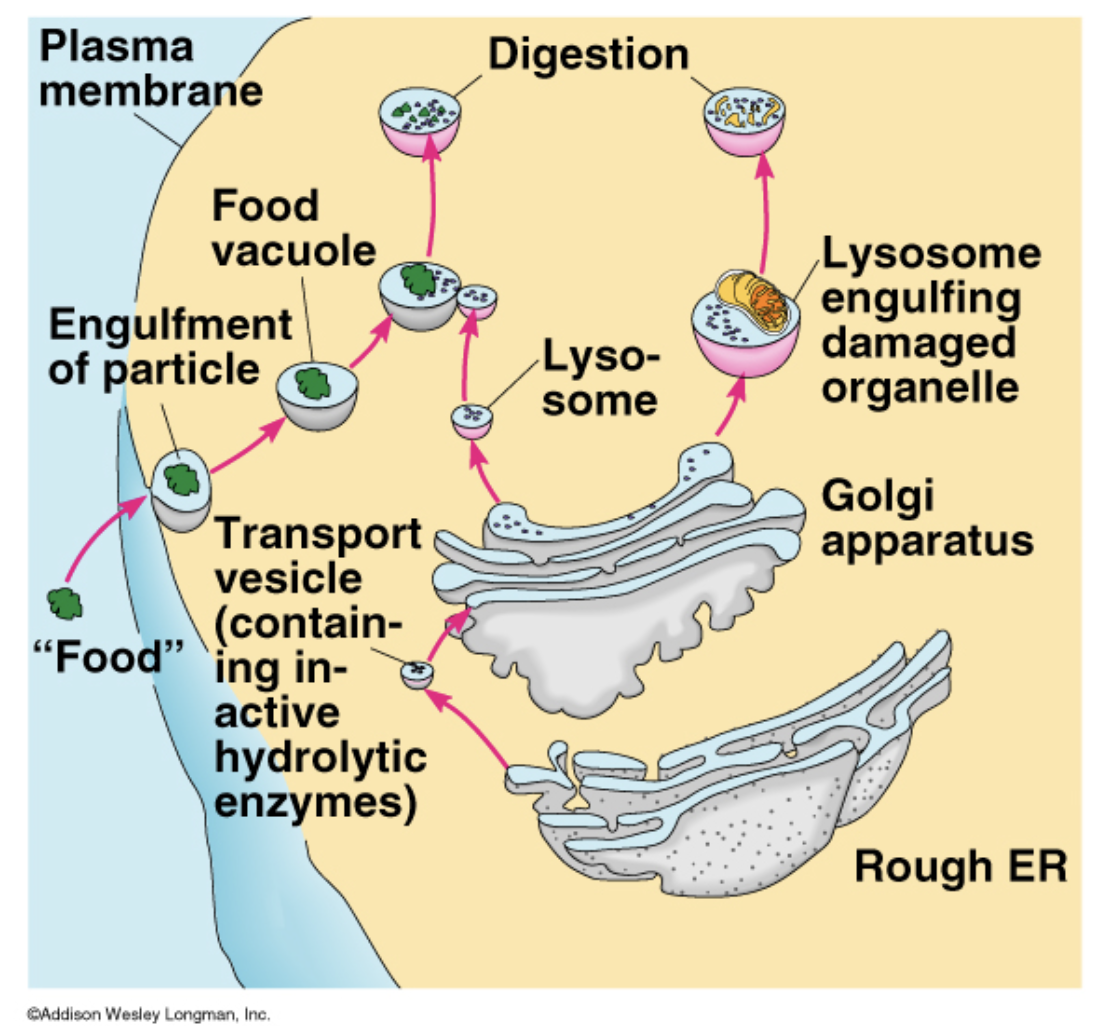
Lysosomes
garbage center
many enzymes designed to break covalent bonds and breakdown very big proteins into smaller pieces
molecules go through many rounds of degredation before being broken down completely to the residues
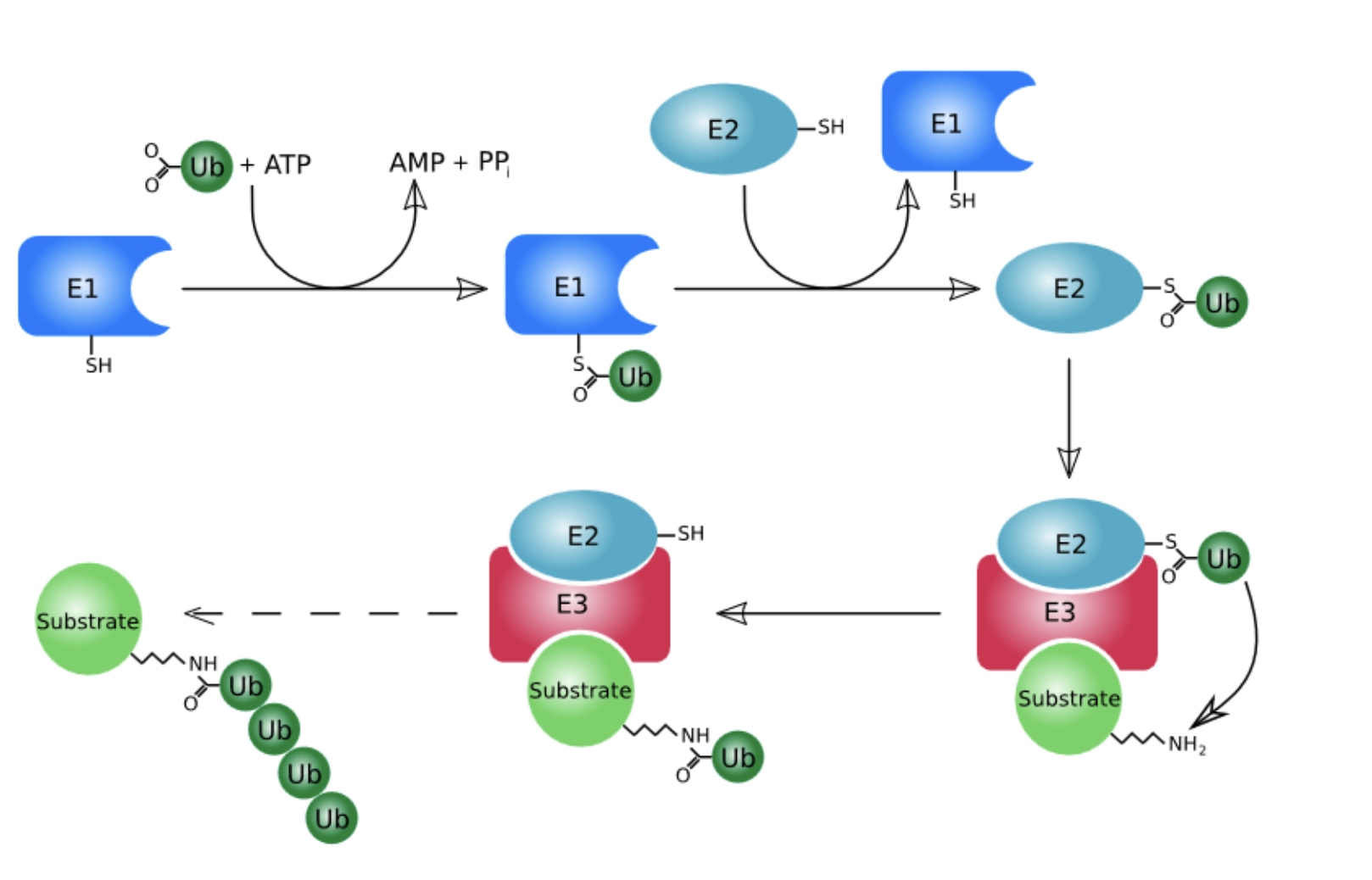
Protein breakdown in eukaryotic cells also occurs in an ___________ that is independent of lysosomes. This process involves ___________: a protein named for its ____________. In order for a protein to be efficiently degraded, it must be _________________. Ubiquitinated proteins are ____________ in an _______________ mediated by a __________________.
ATP-requiring process; ubiquitin; ubiquity and abundance; linked to a chain of at least four tandemly linked ubiquitin molecules; proteolytically degraded; ATP-dependent process; large multiprotein complex named the 26S proteasome
How does the cell know which protein to break down?
ubiquitin marks proteins for degradation
energy cost reaction
Let’s say an enzyme has finished its life cycle, Ubiquitin enzyme attaches to a Ub marker, which then binds to the substrate (protein) and tags it with the Ub marker
we need at least 4 of the markers attached to the substrate before it can be sent to the lysosome for degredation
E1, E2, E3 are essentially just enzymes that aid in adding the Ubiquitin (Ub) marker to the substrate
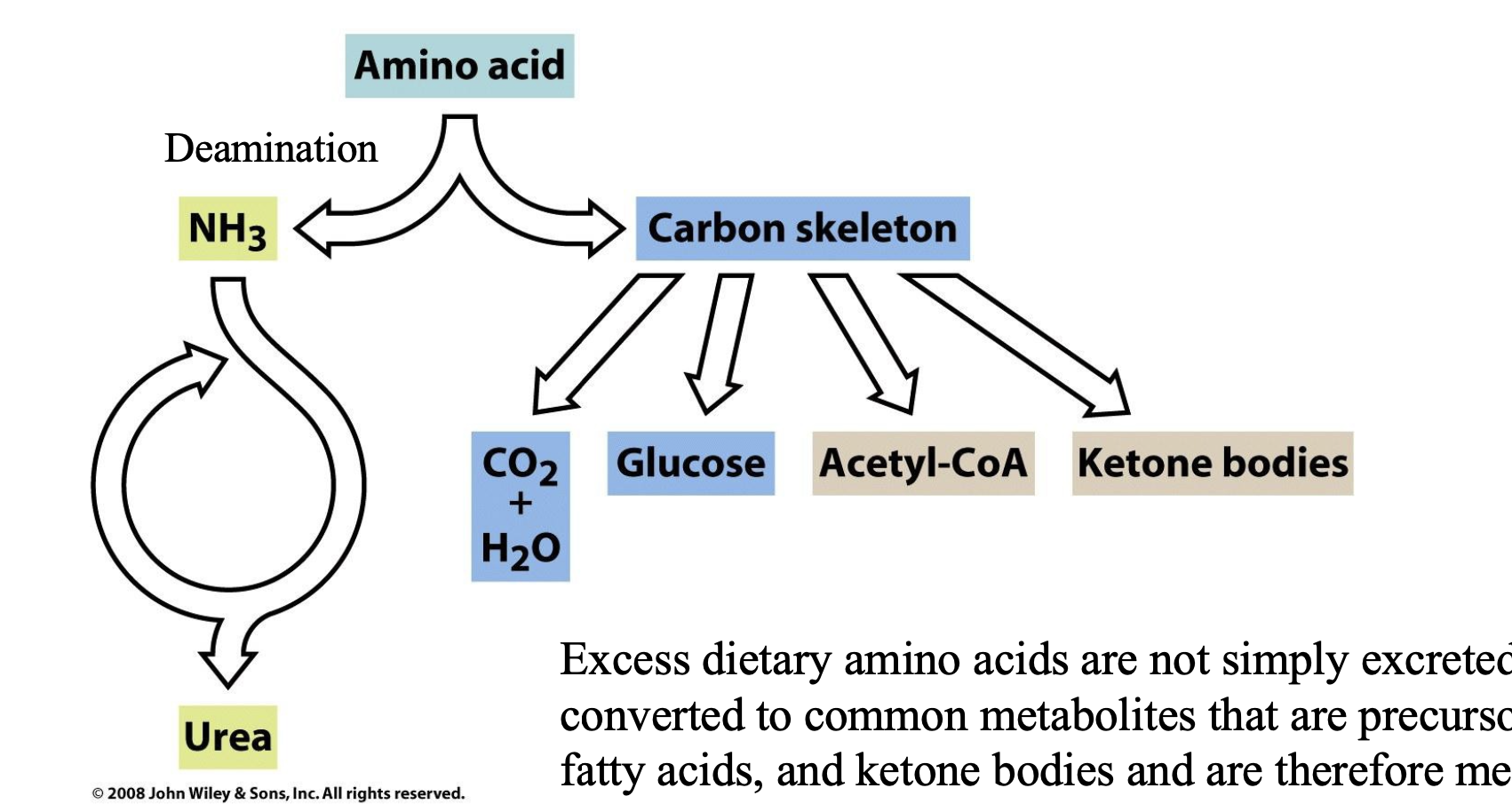
Free amino acids originate from the ______________ and from the ______________. ________________ degrade polypeptides to ____________. These substances are _____________ and ______________ to be _____________. These enzymes are first made in their ___________. For example, _______________ are inactive form of ________________. Synthesis of the enzymes as inactive precursors and the presence of a protein called _____________ protects the pancreas from ______________.
Excess dietary amino acids are not simply excreted but are _________________ that are ____________ and are therefore ______________.
degradation of cellular proteins; digestion of dietary proteins; The gastric protease pepsin, the pancreatic enzymes trypsin, chymotrypsin, and elastase; oligopeptides and amino acids; absorbed by the intestinal mucosa; transported via the bloodstream; absorbed by other tissues; inactive form; pancreatic trypsinogen and chymotrypsinogen; trypsin and chymotrypsin; pancreatic trypsin inhibitor; proteolytic attack.
converted to common metabolites; precursors of glucose, fatty acids, and ketone bodies; metabolic fuels.
How do we deal with the nitrogens?
Build up of N will be toxic for the body
recall there are 20 standard amino acids —> breakdown is focused on these 20 and how to remove the amino groups
once we get rid of the amino group, we are left with carbon skeleton, containing large amounts of energy, and we can convert the carbons to other compounds to supply energy
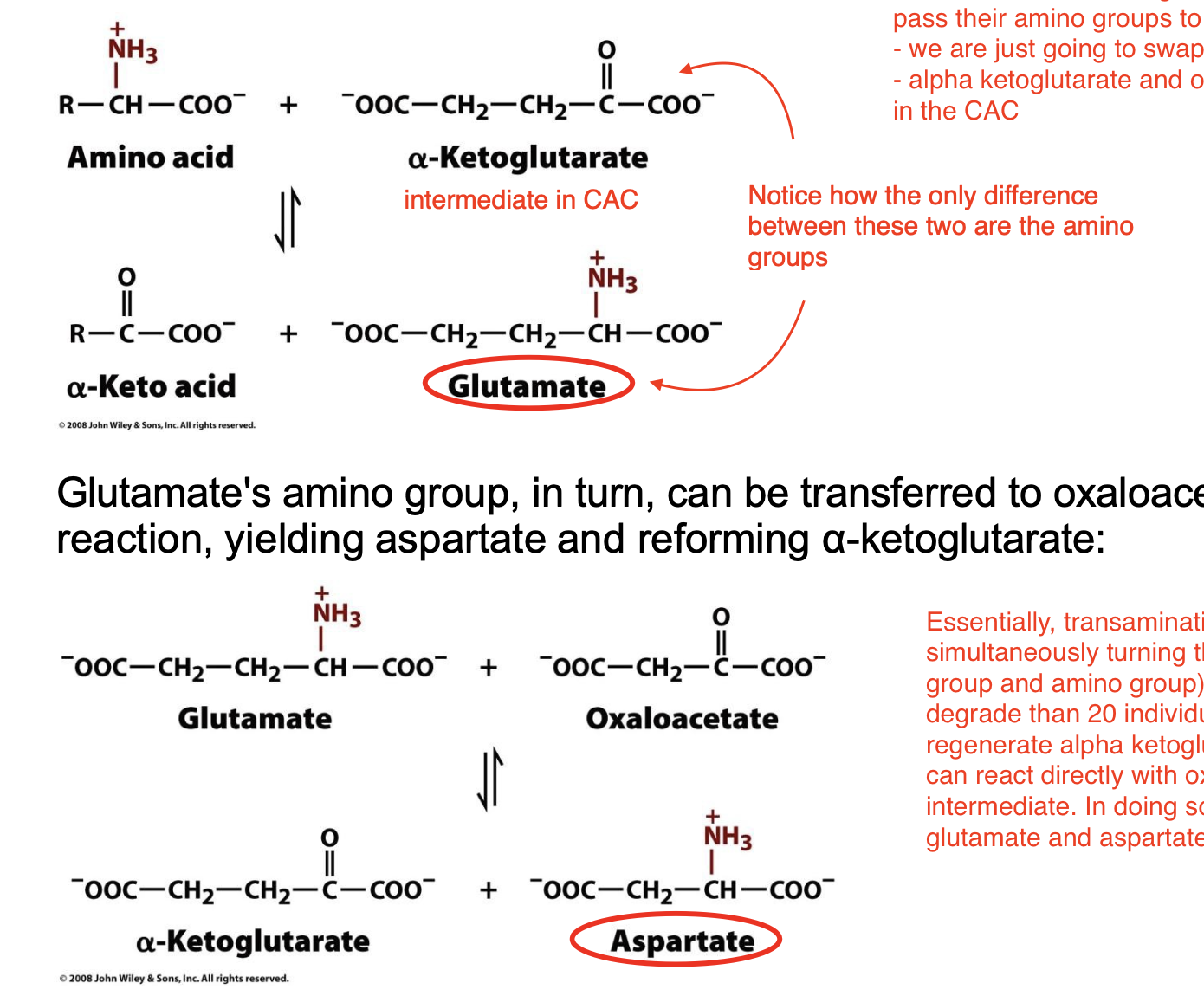
Most amino acids are deaminated by _____________, which can be defined as the ________________. The predominant amino group acceptor is ___________, producing _____________.
Glutamate's amino group, in turn, can be transferred to __________ in a second transamination reaction, yielding ________ and reforming ___________.
transamination; transfer of their amino group to an α-keto acid to yield the α-keto acid of the original amino acid and a new amino acid; α-ketoglutarate; glutamate and the new α-keto acid
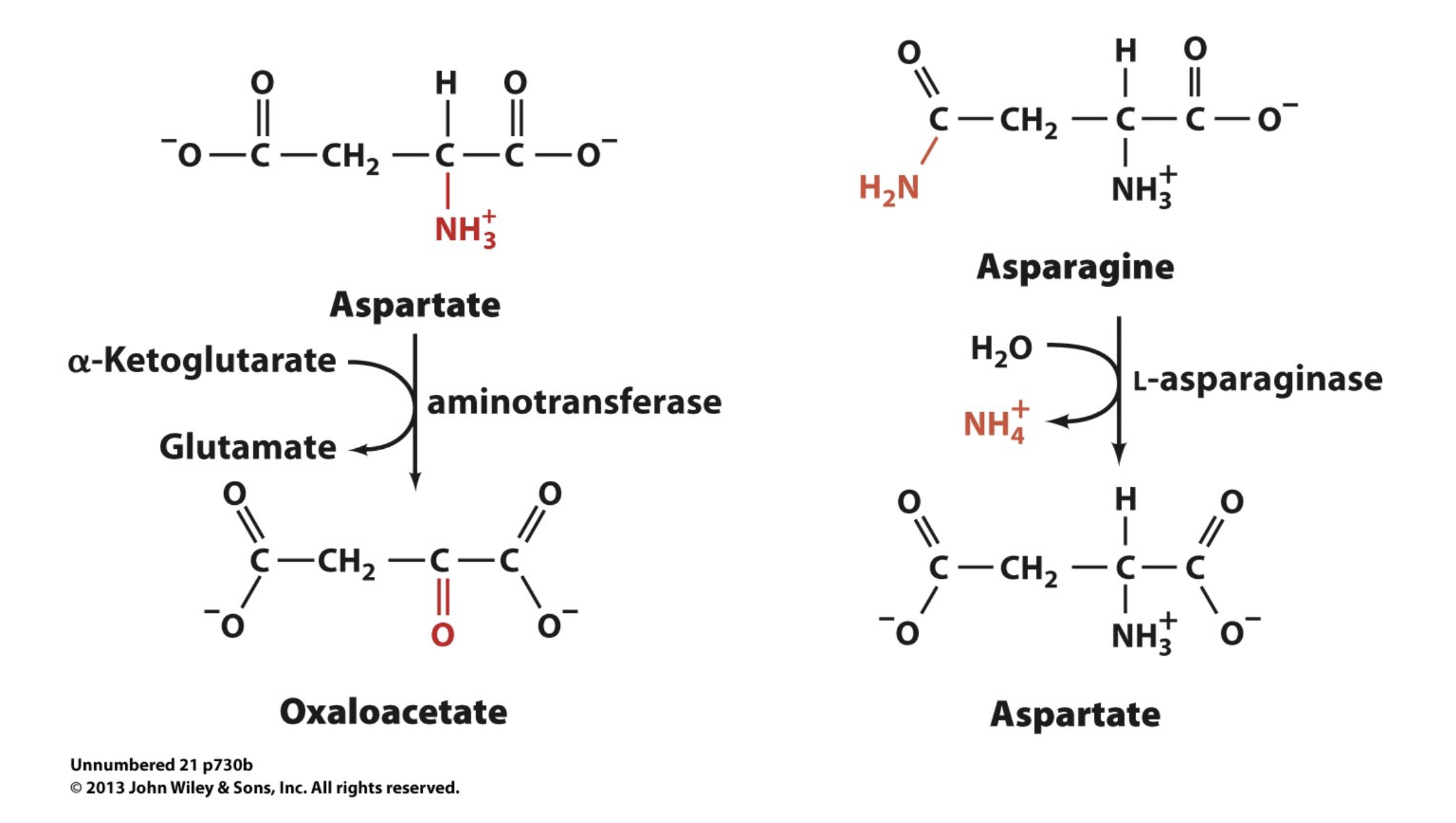
oxaloacetate; aspartate; α-ketoglutarate
Transamination
transamination is a funnel system
instead of deaminating each 20 of the amino acids, we can get each amino acid to pass their amino groups to either 5C alpha ketoglutarate or 4C oxaloacetate
we are just going to swap the functional groups!
alpha ketoglutarate and oxaloacetate are widely available in the cells as intermediates in the CAC
Essentially, transamination converts an amino acid to its alpha keto acid form while simultaneously turning the alpha ketoglutarate into glutamate (we swapped the ketone group and amino group). Then, we now have only glutamates, which is easier to degrade than 20 individual amino acids. So, we can react glutamate with oxaloacetate to regenerate alpha ketoglutarate and convert oxaloacetate to aspartate. Also, amino acids can react directly with oxaloacetate to form aspartate, skipping the glutamate intermediate. In doing so, we ensure that all amino acids are concentrated to only glutamate and aspartate, for easier removal from the body.
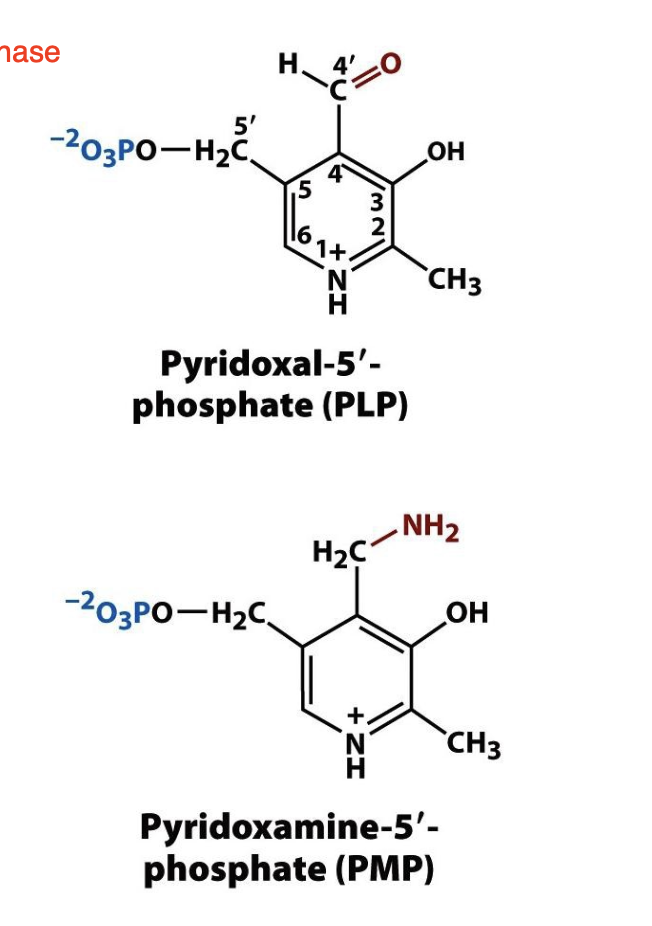
The enzymes that catalyze transamination, called _______________, require the coenzyme _____________.
aminotransferases or transaminases; pyridoxal-5′-phosphate (PLP)
What is important to note about aminotransferases?
Aminotransferases differ in their specificity for amino acid substrates in the first stage of the transamination reaction, thereby producing the correspondingly different α-keto acid products.
Most aminotransferases, however, accept only α-ketoglutarate or (to a lesser extent) oxaloacetate as the α-keto acid substrate in the second stage of the reaction, thereby yielding glutamate or aspartate as their only amino acid product.
The amino groups from most amino acids are consequently funneled into the formation of glutamate and aspartate.
The presence of transaminases in muscle and liver cells makes them _____________. Assays of the enzymes' activities in the blood are the ______________ known as _____________ and ______________. The concentrations of these enzymes in the blood ___________ after a heart attack, when damaged heart muscle _______________. Liver damage is also monitored by _______________.
useful markers of tissue damage; basis of the commonly used clinical measurements; SGOT (serum glutamate-oxaloacetate transaminase, also known as aspartate transaminase, AST); SGPT (serum glutamate-pyruvate transaminase, or alanine transaminase, ALT); increase; leaks its intracellular contents; SGOT and SGPT levels.
Structure of PLP and PMP
Transamination, of course, does not result in any ____________. Glutamate, however, can be ___________ by _____________, yielding ___________ and regenerating _____________ for use in additional _____________.
Thus, the glutamate dehydrogenase reaction functions to _________________.
net deamination; oxidatively deaminated; glutamate dehydrogenase (GDH); ammonia; α-ketoglutarate; transamination reactions.
… eliminate amino groups from amino acids that undergo transamination reactions with α-ketoglutarate.
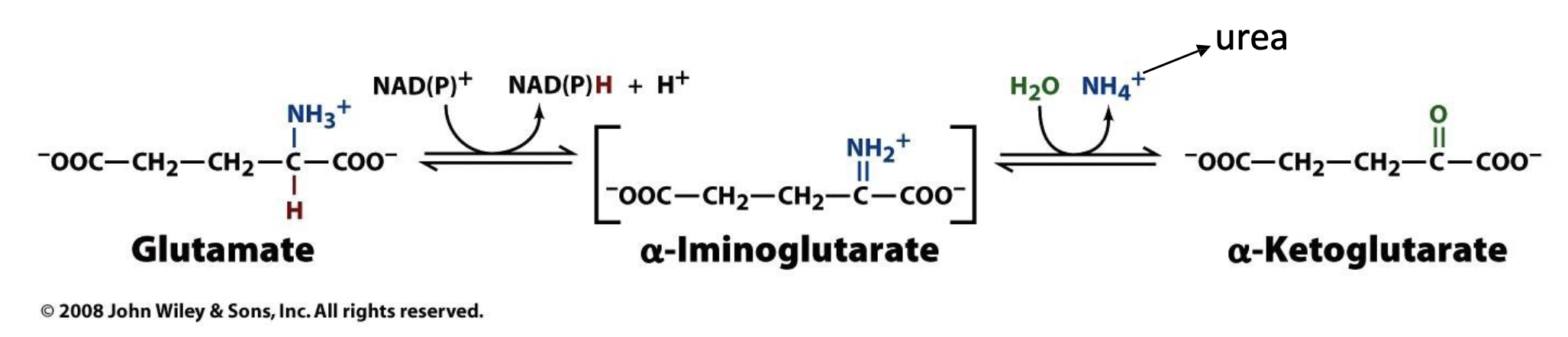
Explain in detail the first step of the urea cycle
In the first step of the urea cycle, we must deaminate glutamate, this occurs via enzyme glutamate dehydrogenase and an electron carrier (NAD+ but in some organisms it uses NADP+). Electron carrier helps oxidize glutamate and release the amino group while converting glutamate back to alpha ketoglutarate. However, since the amino group is released as ammonia (NH4+), this is not very stable and can easily decompose to NH3 and H+. The NH3 is what participates directly in the urea cycle! Note that the alpha ketoglutarate we generated can go back and be used to convert amino acids again!
Ammonia is quite ____________. The ammonia intoxication in humans is characterized by an ____________. This may have been caused by the ________________, which acts as an ________________.
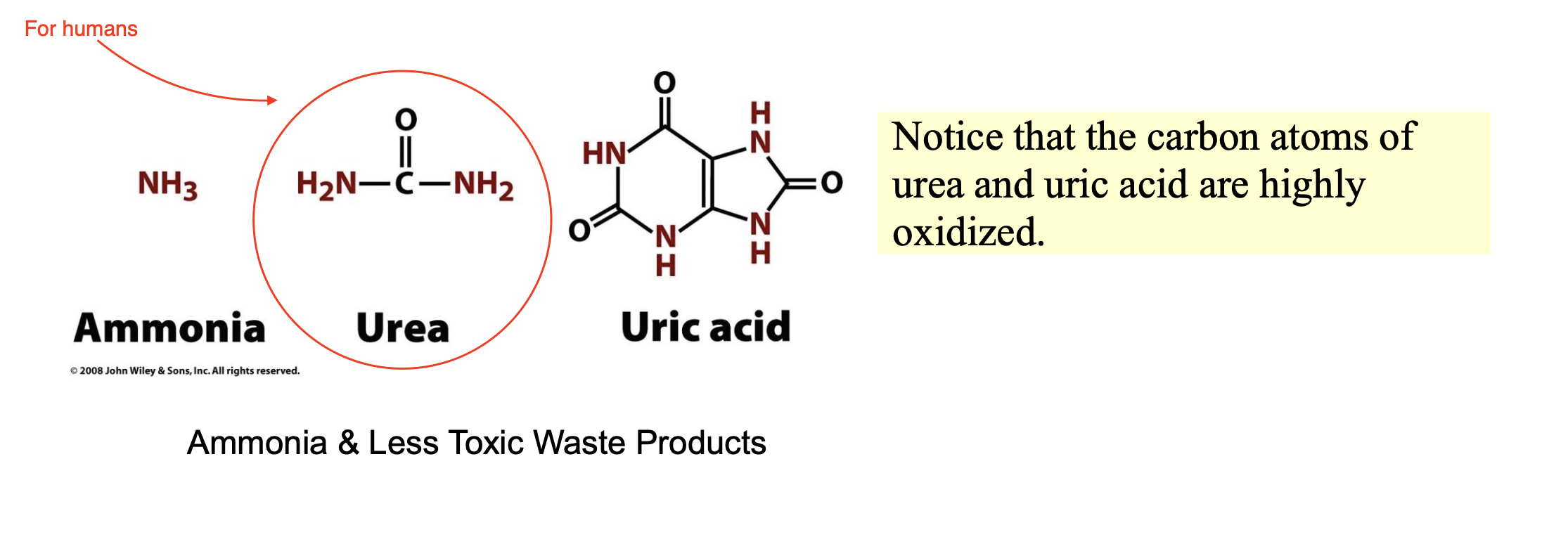
If not reused for the synthesis of new amino acids or other nitrogenous products, excess NH4+ is excreted as __________ (microbes and fish), ________ (most terrestrial vertebrates), or _________ (birds and terrestrial reptiles). Urea is synthesized in the _________ by the ____________. It is then __________ and sequestered by the ______ for ____________.
toxic to animal tissues; increase in the brain’s water content; glutamine synthetase reaction making a high levels of glutamine; osmotically active solute.
ammonia; urea; uric acid; liver; enzymes of the urea cycle; secreted into the bloodstream; kidneys; excretion in the urine.
How can nitrogen be released from the body?
depends on the organism
Urea group is a very efficent molecule to get rid of nitrogen bc it can hold 2 amino groups at once
birds use uric acid which can contain many nitrogens at once
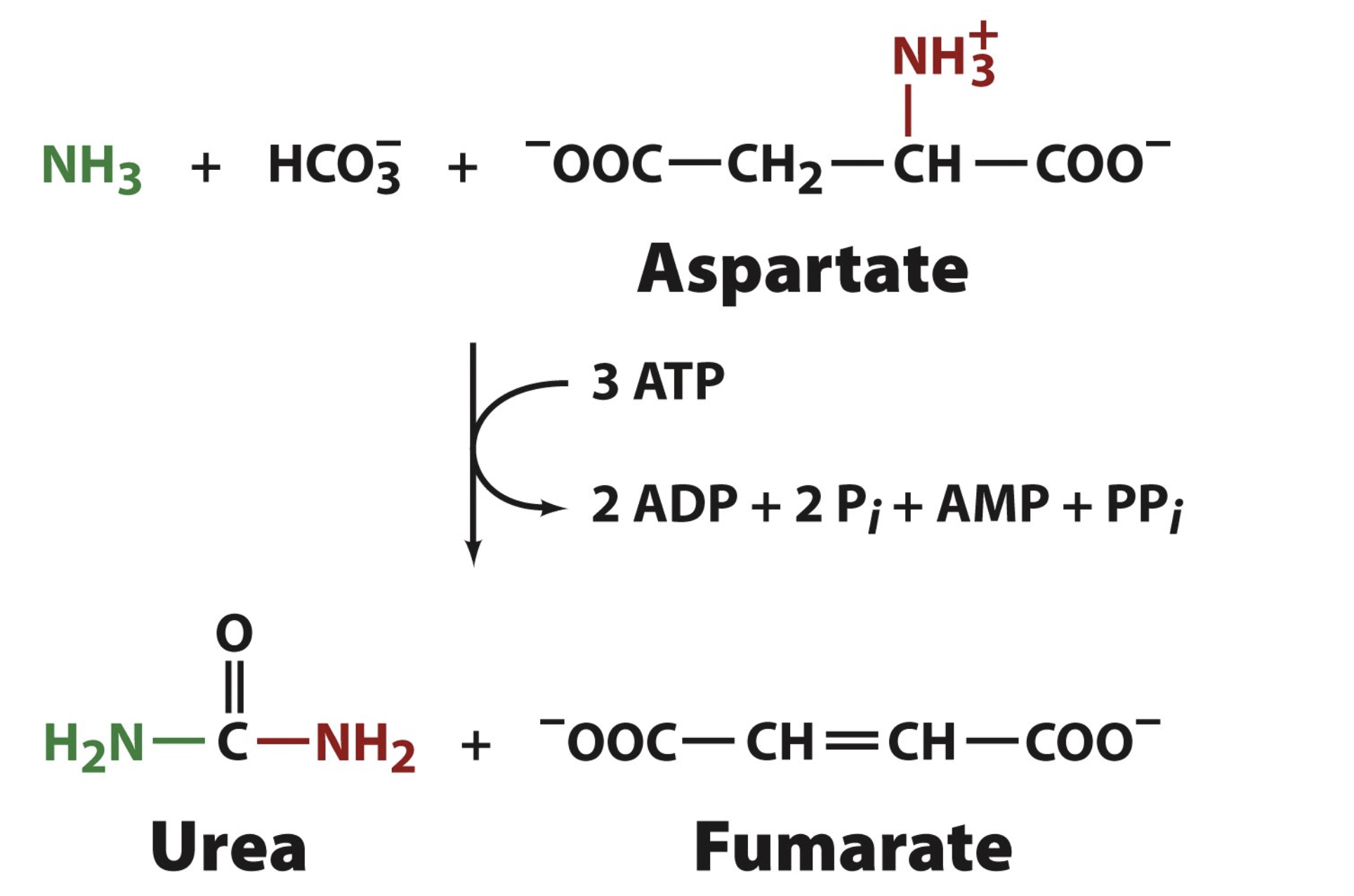
Overall Urea Cycle Reaction
Urea Cycle —> how we make urea
5 steps to complete one cycle
one cycle produces one urea
from transamination, we know that amino groups are past to aspartate and glutamate, so aspartate and glutamate each donate their amino group to urea through the urea cycle (this is where the 2 amino groups on urea come from)
Give an Overview of the Urea Cycle
takes 5 steps per cycle
reactions 1 & 2 occur in mitochondria and the rest of the reactions in the cytosol
recall we said that one of the amino groups comes from aspartate and the other comes from glutamate
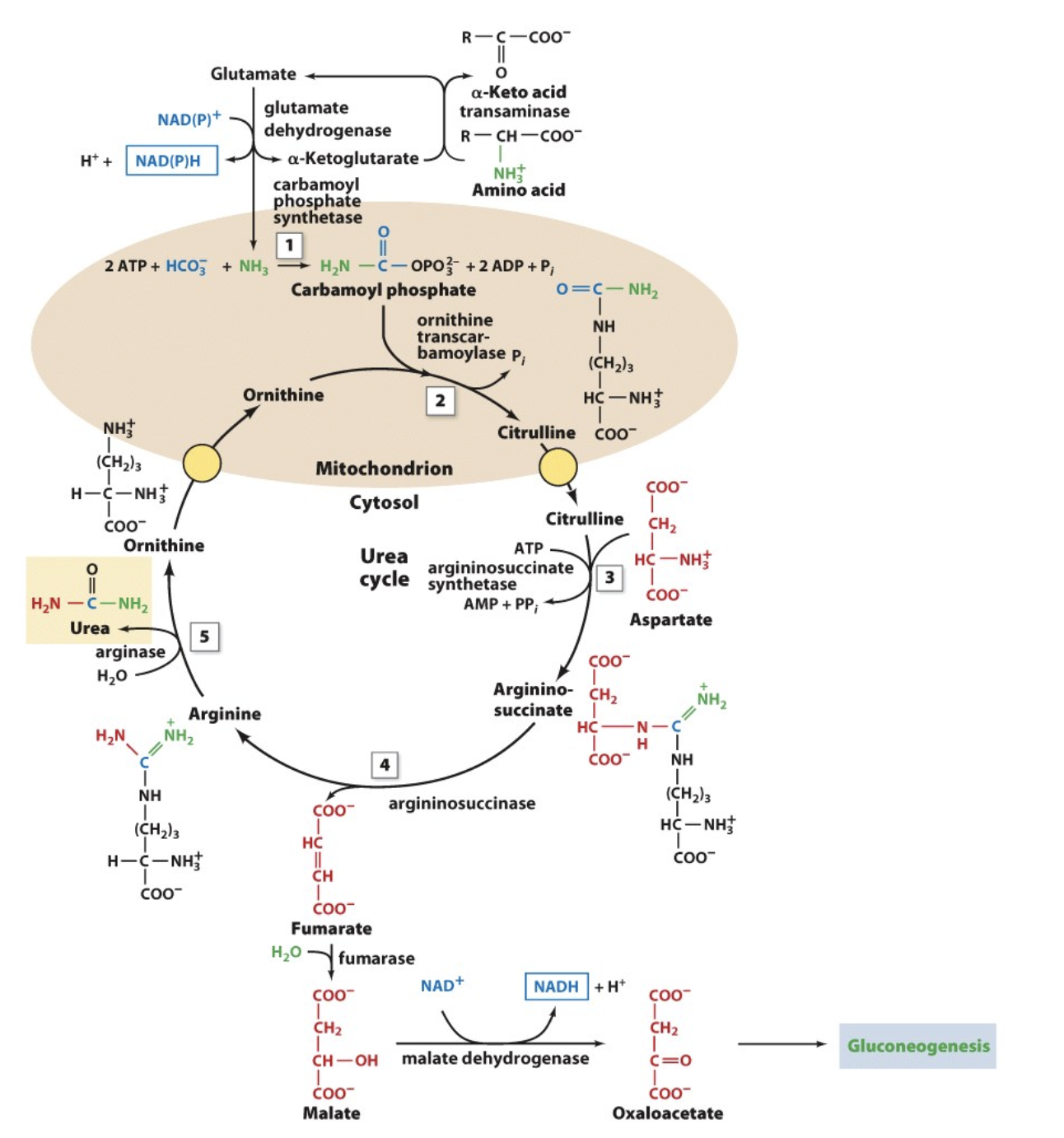
The Urea Cycle: Step 1
glutamate converted to alpha ketoglutarate via a dehydrogenase reaction, releasing an amino group
amino group reacts with bicarbonate and 2 ATP to give carbamoyl phosphate
this bicarbonate is the one to supply the central carbon of the urea molecule
note that carbon dioxide is what supplies the bicarbonate, because when the CO2 is dissolved in water in the body it converts to bicarbonate
note that an inorganic phosphate group is added to stabilize the compound and thats why we need two ATP (one for energy supply and the other as a stabilizer)
the remaining 4 steps of the cycle is to replace the phosphate group with the second amino group (from aspartate)
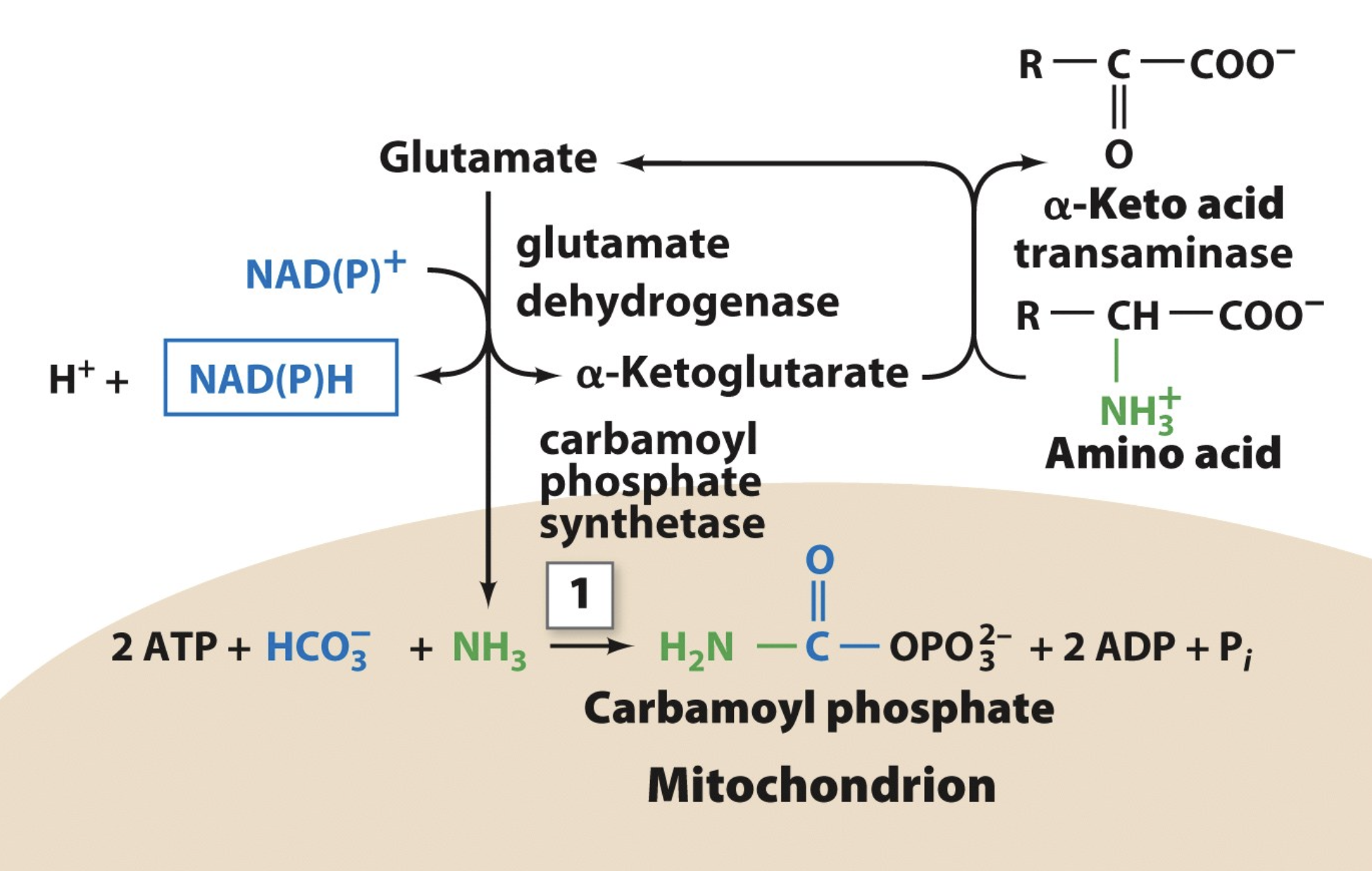
The Urea Cycle: Step 2
occurs between carbamoyl phosphate and ornithine
releases inorganic phosphate
remaining molecule connects with ornithine (called citrulline)
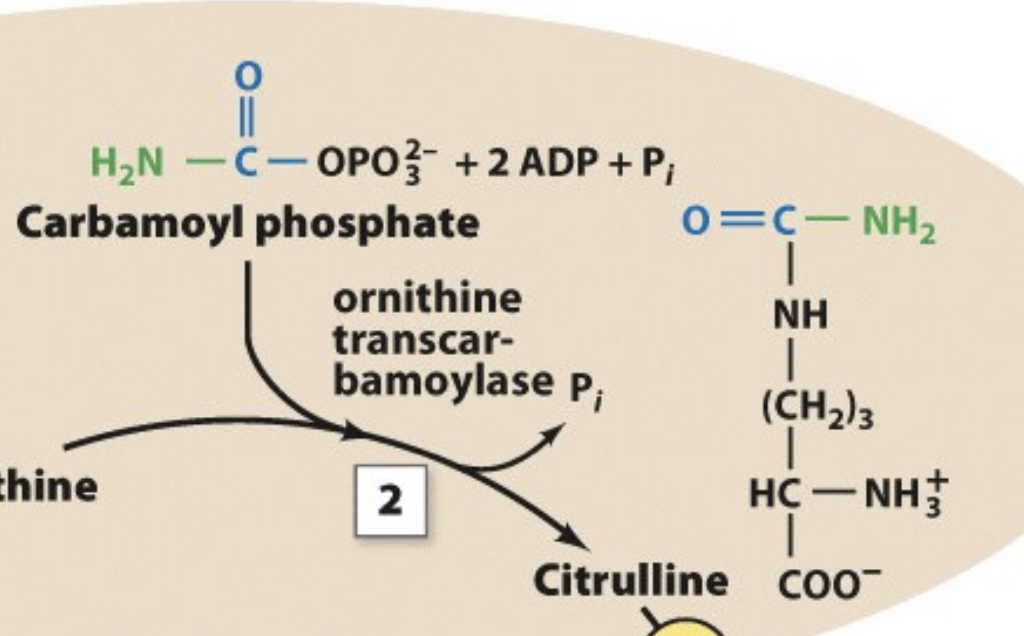
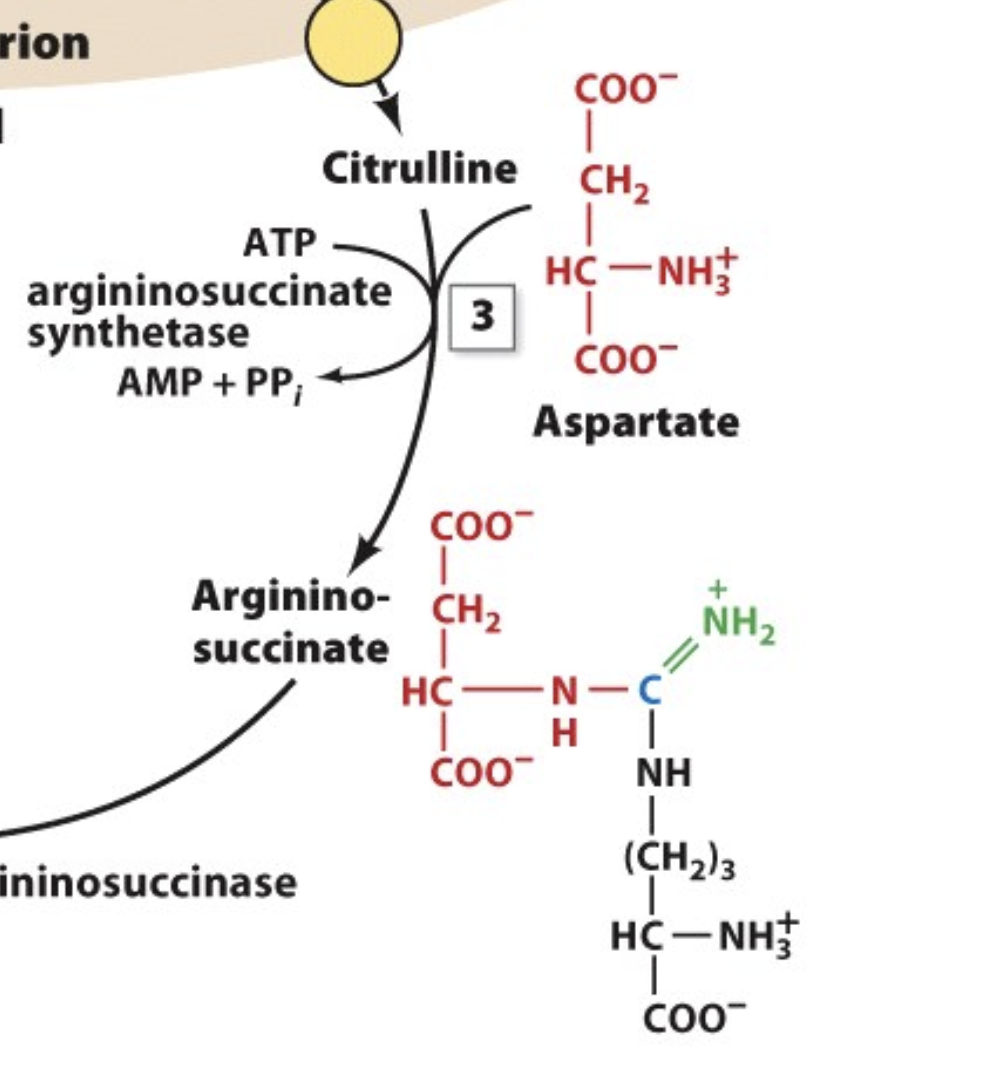
The Urea Cycle: Step 3
aspartate can now react with the citrulline to form an even bigger molecule called arginino-succinate
the carbon of citrulline connects to aspartate via the amino group on aspartate
this reaction requires the input of energy (ATP)
However, converting ATP to ADP is not enough, we need to convert ATP to AMP which releases double the amount of energy!
so technically, the energy cost is equivalent to 2 ATPs
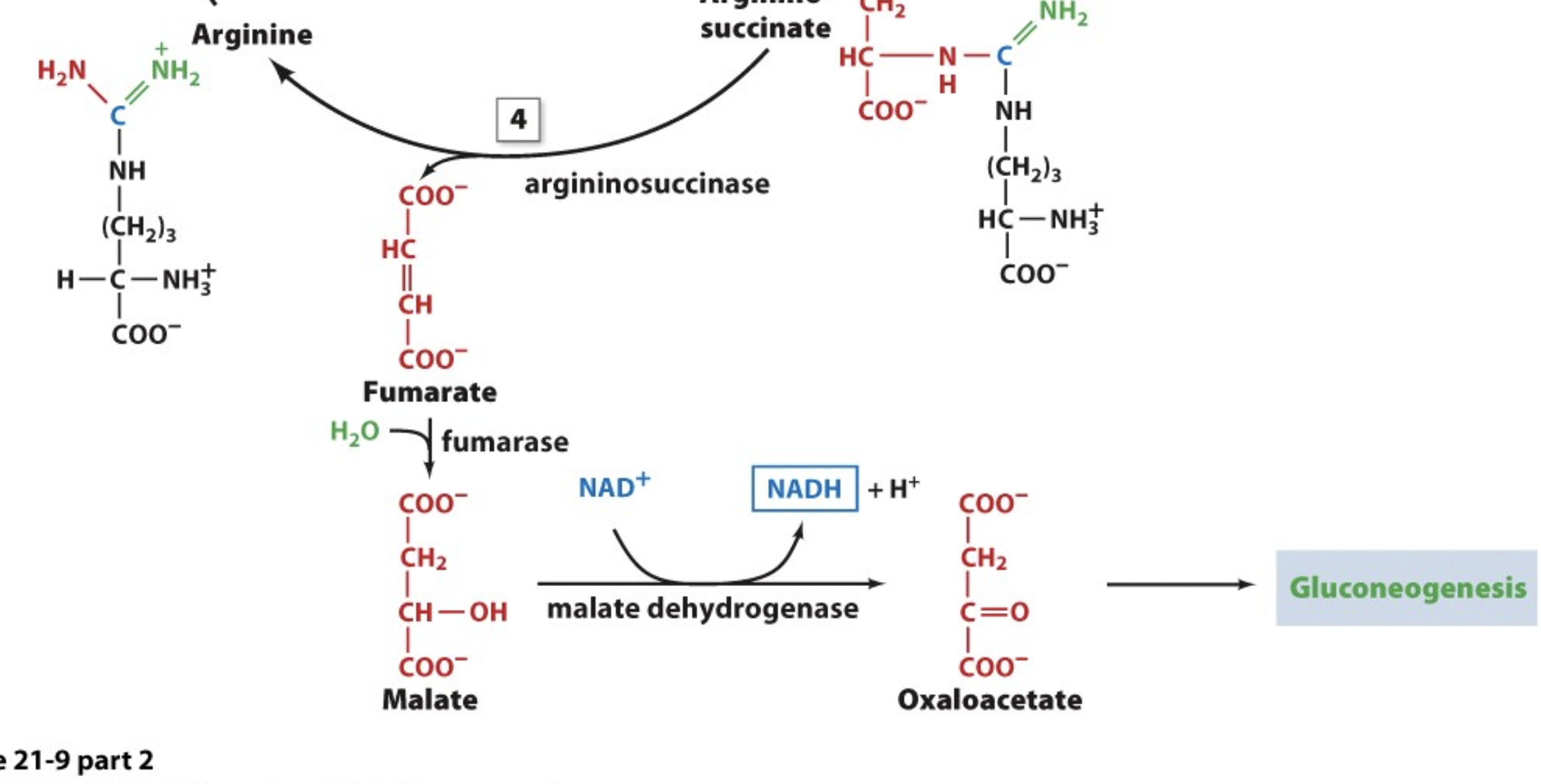
The Urea Cycle: Step 4
so now we have to break the bond between NH and the succinate group from argininosuccinate
argininosuccinase is the enzyme for this break
the succinate group can then be converted to fumarate, then malate, then oxaloacetate and go through gluconeogenesis
what we have remaining is essentially arginine!
The Urea Cycle: Step 5
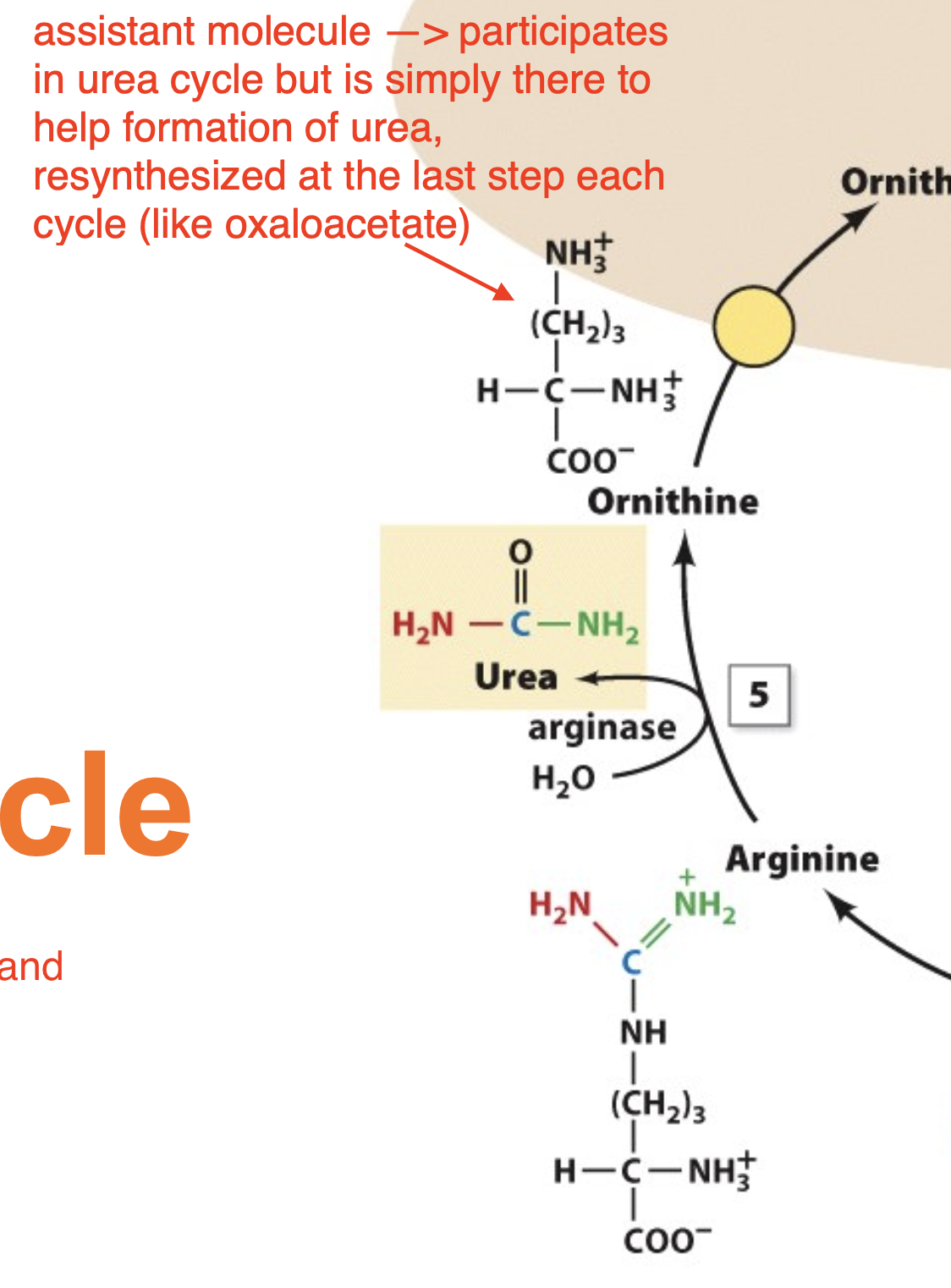
We are going to break arginine using water and arginase to give us urea
the remaining compound is actually our assistant molecule from the beginning —> ornithine
Explain the role of ornithine
assistant molecule —> participates in urea cycle but is simply there to help formation of urea, resynthesized at the last step each cycle (like oxaloacetate)
Where does the Urea Cycle happen
happens primarily in the liver and kidney cells (majority in liver and are transported to kidney)!
Urea cycle – Review (sequence of reactions)
Blue = main compounds in cycle
Red = enzymes for each step

The overall chemical balance of the biosynthesis of urea
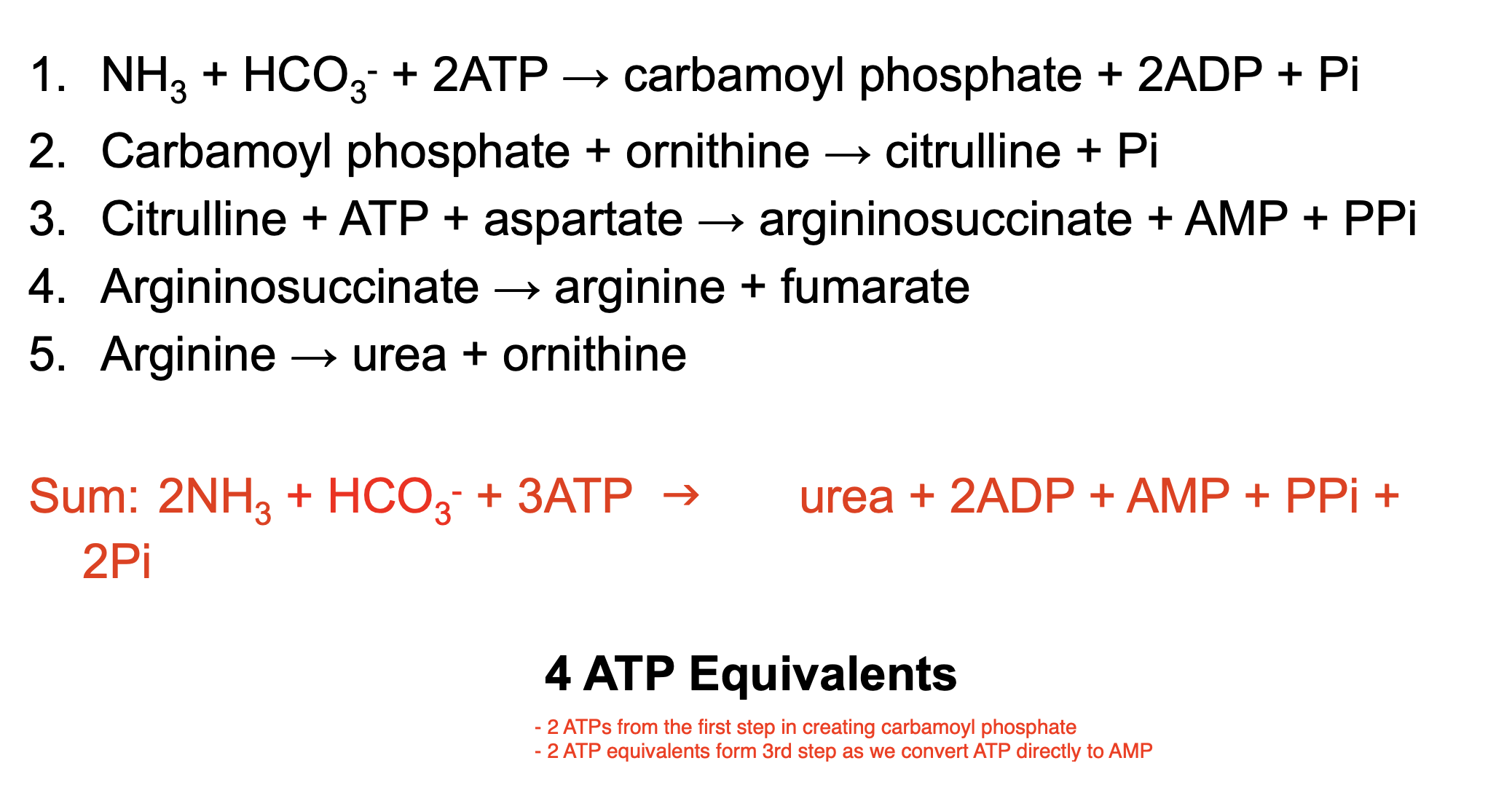
Where does the 4 ATP equivalents come from?
2 ATPs from the first step in creating carbamoyl phosphate
2 ATP equivalents form 3rd step as we convert ATP directly to AMP
We talked about where the amino group goes from the amino acid breakdown, but what about the remaining carbon skeleton?
we can convert carbon skeleton to glucose, acetyl CoA, or ketone bodies (recall ketone bodies are alternate fuel molecule to replace glucose)
How are ketone bodies synthesized?
beta oxidation turns fatty acids to acetyl CoA, which can be used to create ketone bodies
acetyl CoA is 2C, so 2 acetyl CoA forms 4C ketone body
so ketone bodies are essentially the storage from of acetyl CoA
Amino acids are degraded to compounds that can be metabolized to _____________ or used in ______________. Oxidative breakdown of amino acids typically accounts for ________ of the metabolic energy generated by animals.
CO2 and H2O; gluconeogenesis; 10 to 15%
Glucogenic vs Ketogenic Amino Acids
Glucogenic amino acids, which are degraded to pyruvate, α-ketoglutarate, succinyl-CoA, fumarate, or oxaloacetate and are therefore glucose precursors.
Ketogenic amino acids, which are broken down to acetyl-CoA or acetoacetate and can thus be converted to fatty acids or ketone bodies.
Explain the breakdown of amino acids
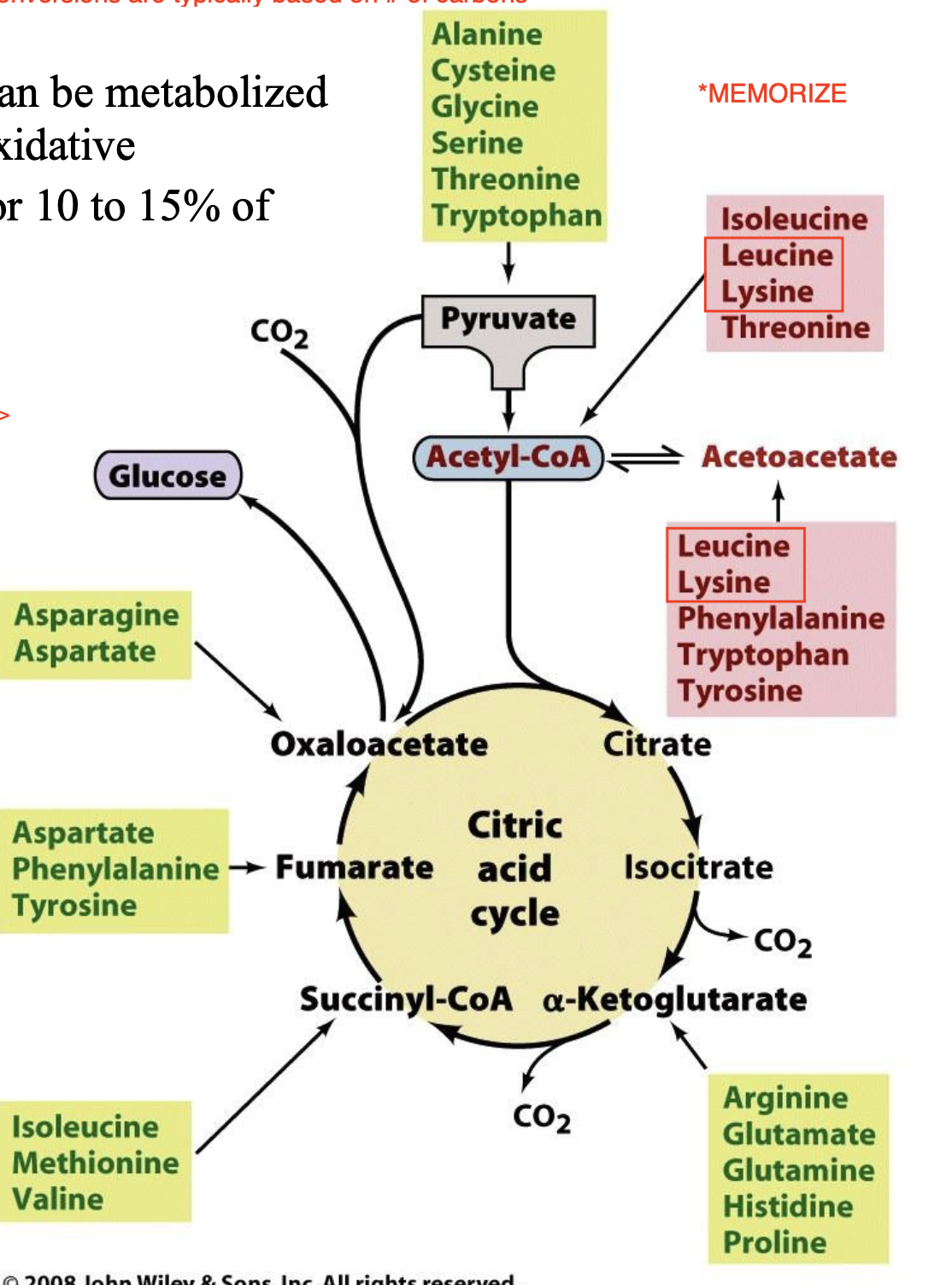
after amino group is removed, the remaining carbon skeleton from the amino acids can be used to create many different molecules
diagram shows the final product after the conversion, each box shows exactly which amino acid can be converted to what compound
green boxes are glucogenic amino acids —> the carbon skeletons of the amino acids in the green boxes create compounds that can be incorporated into gluceonogenesis to give glucose
red boxes are ketogenic amino acids —> the carbon skeletons of the amino acids in the red boxes create either acetoacetate (type of ketone body) or acetyl CoA, eventually synthesizing ketone bodies & fatty acids (recall acetyl CoA cannot be converted back to pyruvate and thus cannot participate in gluceonogenesis)
*Notice how there is an overlap in the green/red boxes, these amino acids can be used to synthesize ketone bodies and glucose
in humans, only two amino acids are exclusively ketogenic —> leucine and lysine
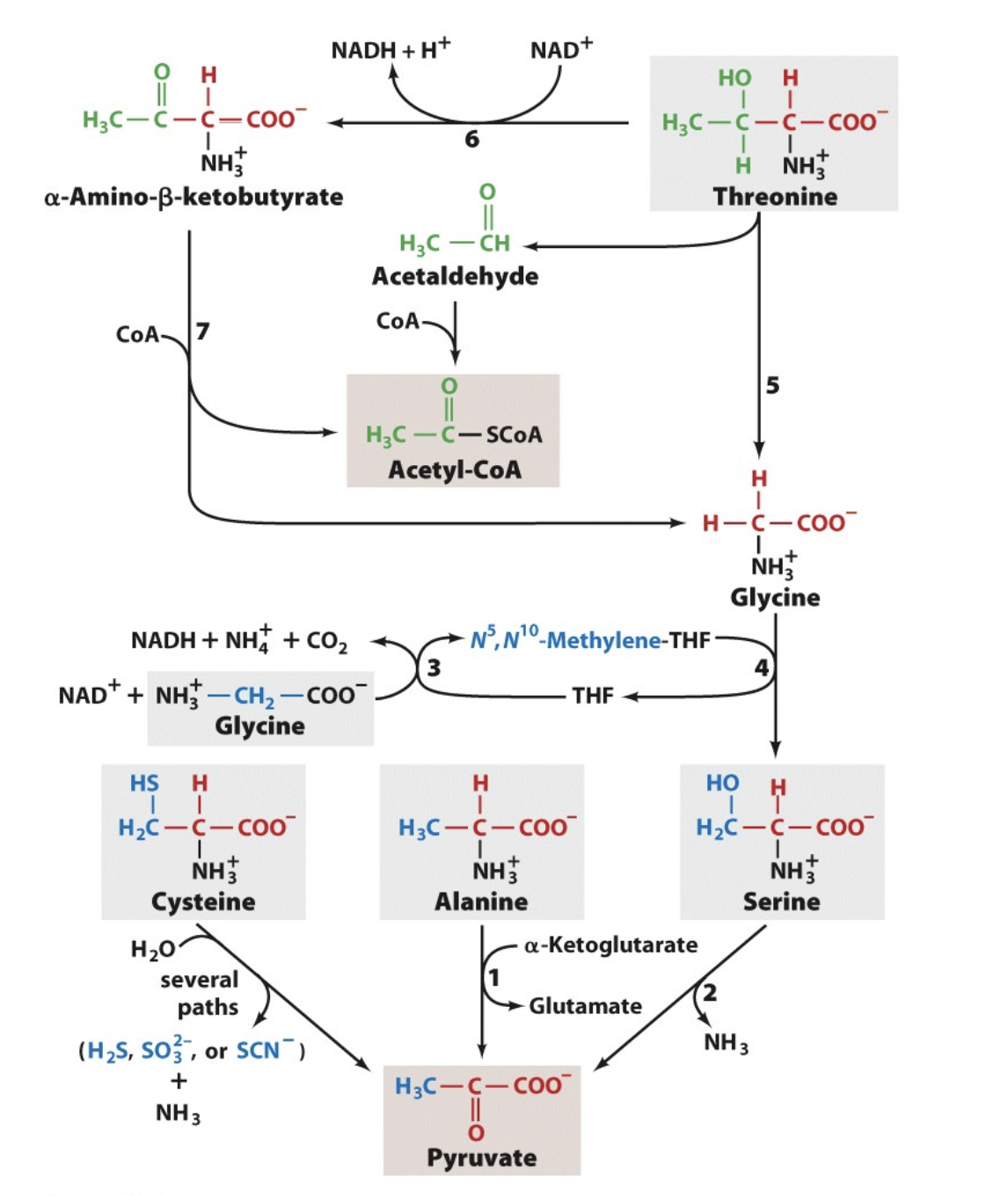
A, C, G, S & T to Pyruvate
notice how cysteine, alanine, and serine are 3C molecules and can be converted to 3C pyruvate!
Asn & Asp to Oxaloacetate
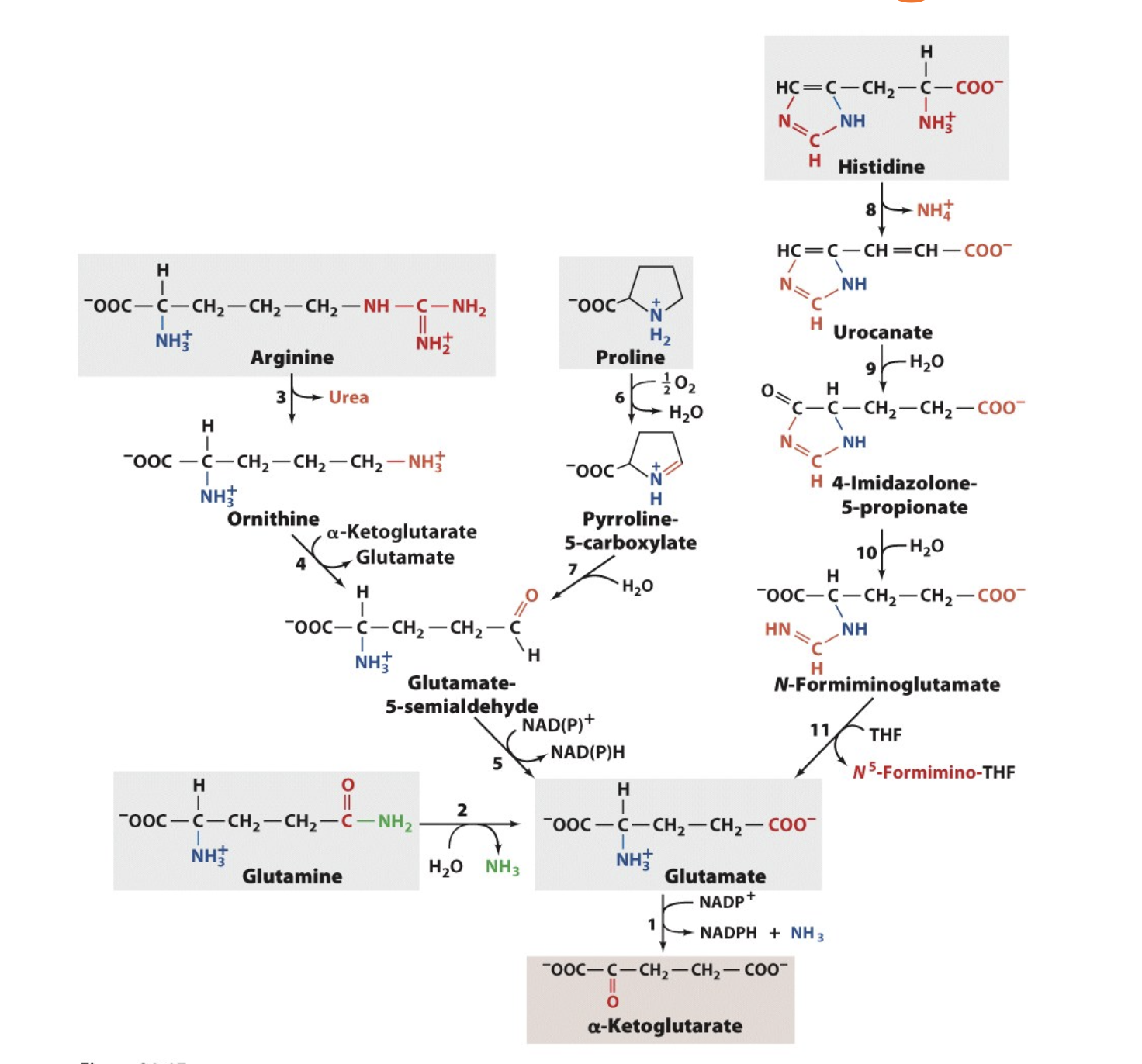
R, E, Q, H, and P to α-Ketoglutarate
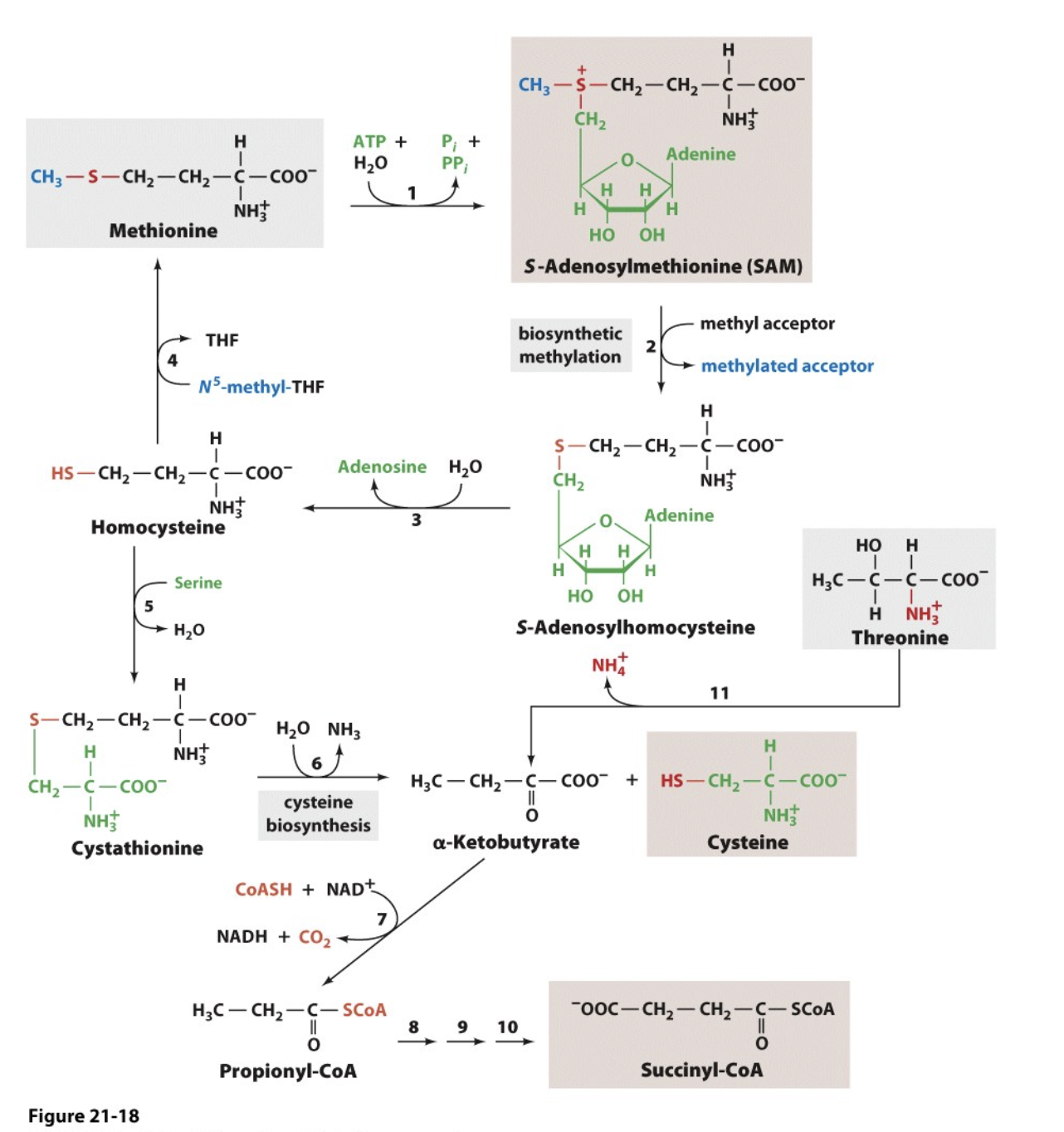
Met & Thr Degradation
Lysine Degradation

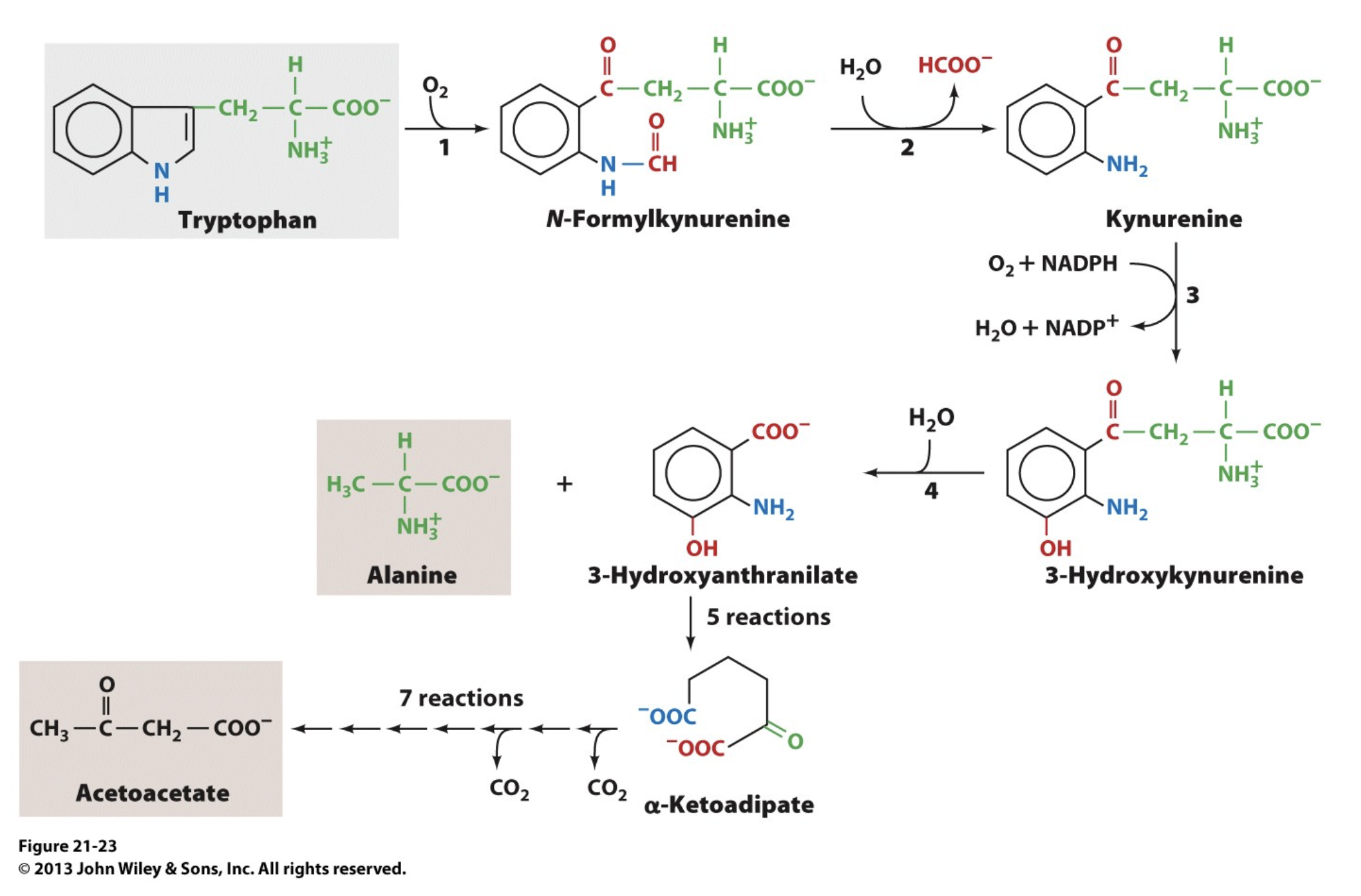
Tryptophan Degradation
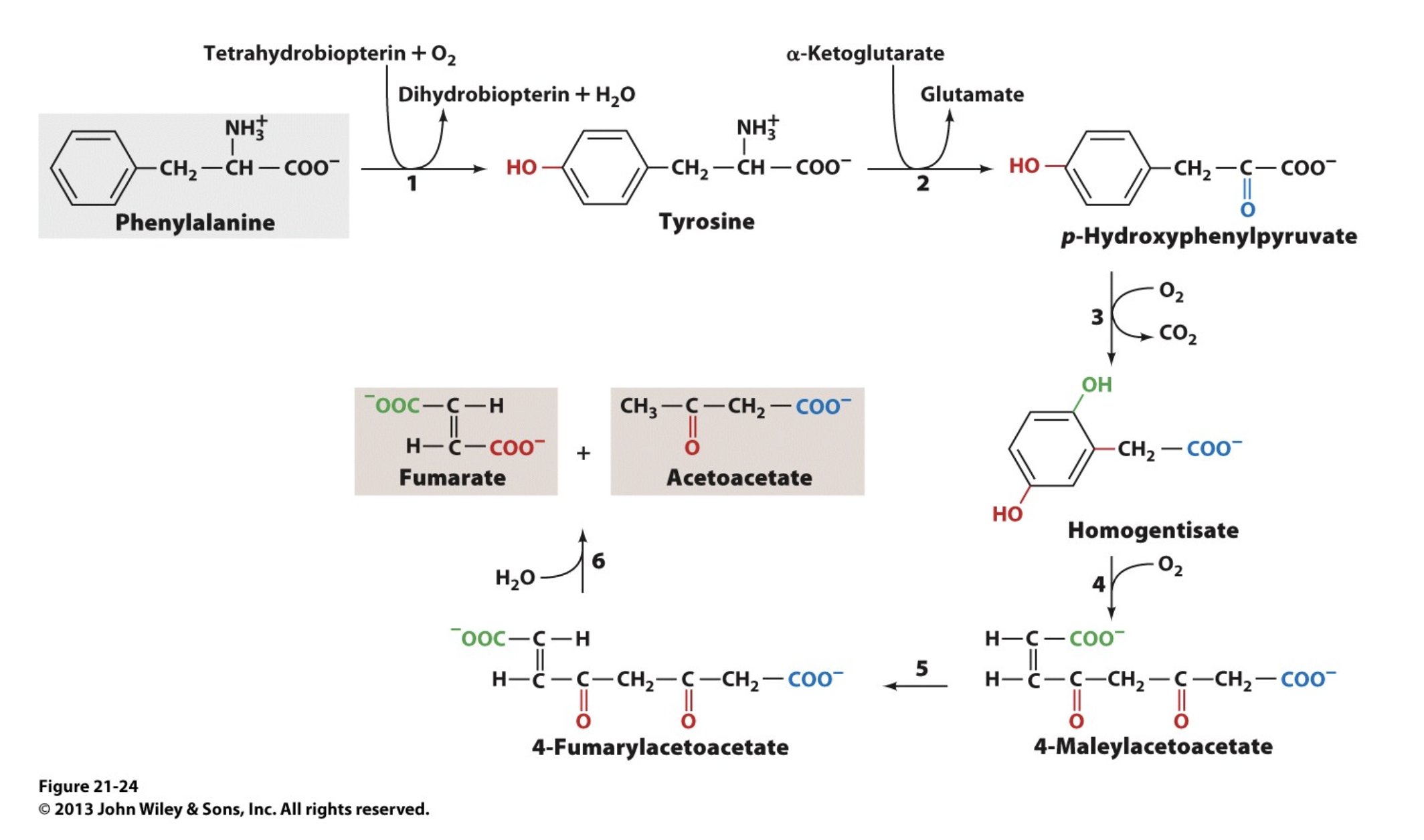
Phenylalanine Degradation
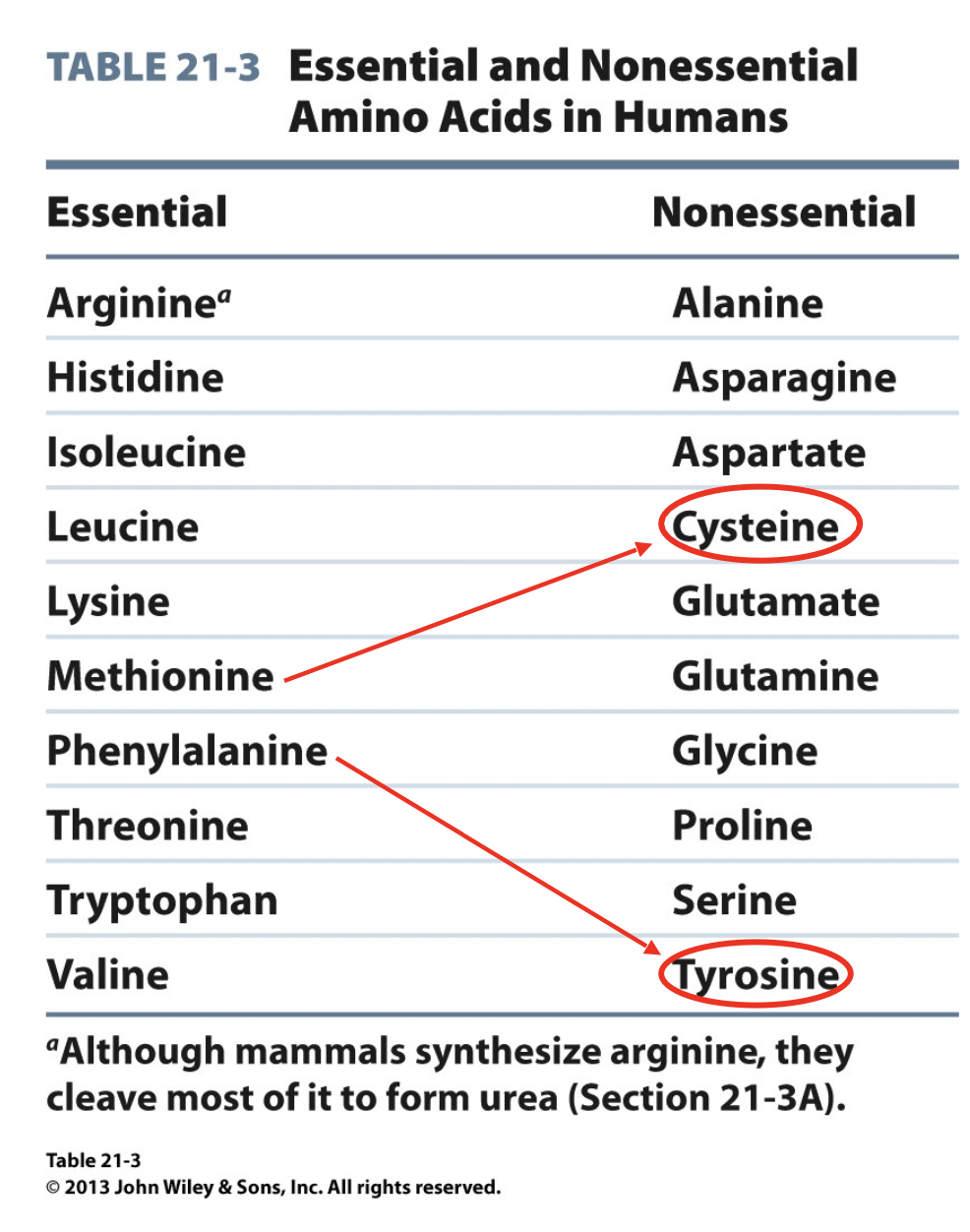
Many amino acids are synthesized by ______________. Since mammals must obtain these amino acids in their diets, these substances are known as _____________. The other amino acids, which can be synthesized by mammals from common intermediates, are termed ____________. These two categories are _______________.
pathways that are present only in plants and microorganisms; essential amino acids; nonessential amino acids; another way to classify amino acids
Essential vs. Nonessential Amino Acids
we already discussed glucogenic amino acids and ketogenic amino acids
another way is essential vs. nonessential
not all amino acids can be synthesized in the body, some must be brought into the body via the food we eat
essential amino acids are the ones we MUST consume in order to have
nonessential are synthesized in our body
*note that even though cysteine and tyrosine are nonessential and can be synthesized in the body, they require the raw material of methionine and phenylalanine (respectively) to be produced
The ultimate source of the α-amino group in these transamination reactions is __________, which is synthesized in _____________ by ___________, which is ____________.
glutamate; microorganisms, plants, and lower eukaryotes; glutamate synthase; enzyme that is absent in vertebrates
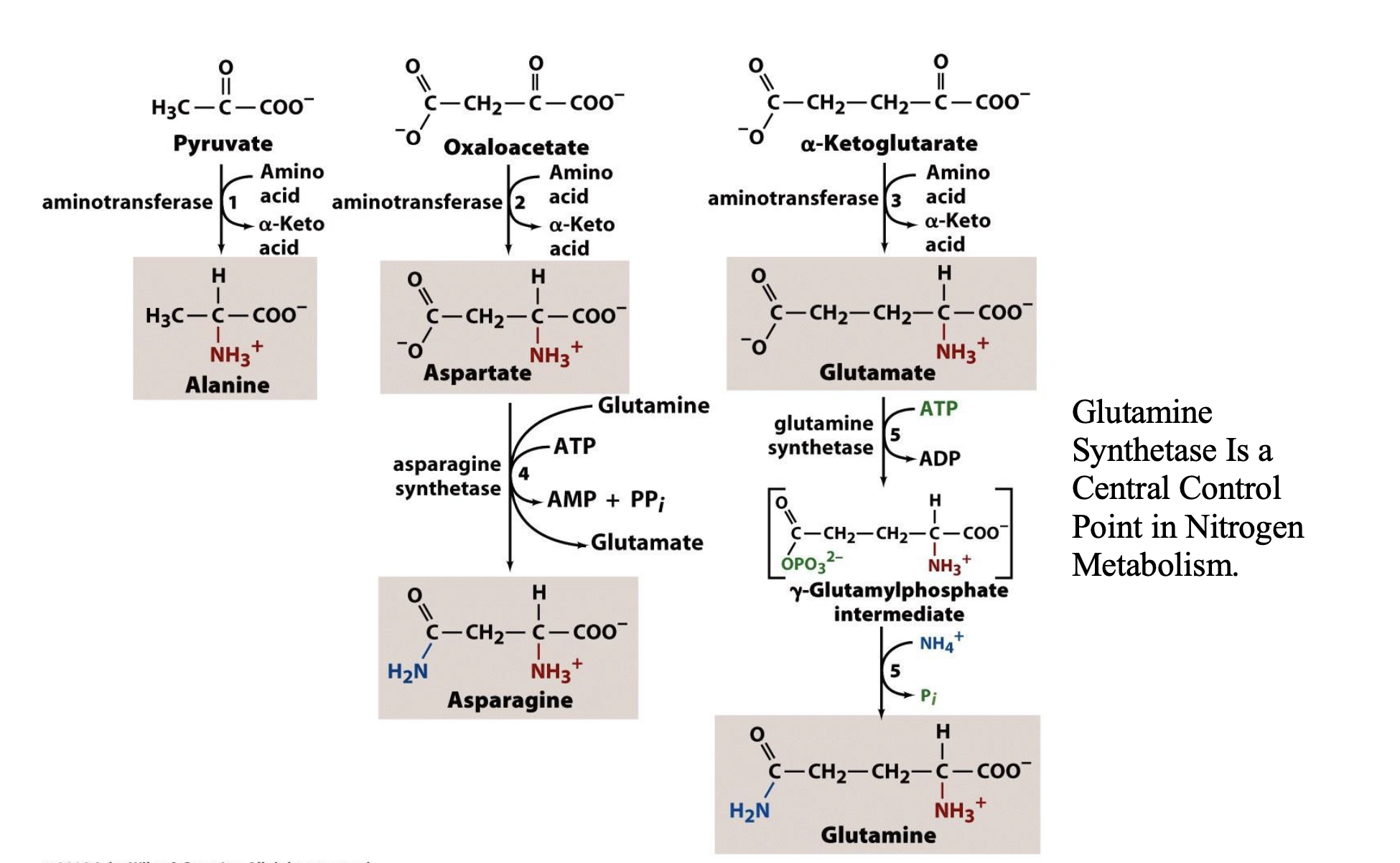
Glutamine Synthetase — why is it important in nitrogen metabolism?
transamination is the method by which the nonessential amino acids are synthesized in the body
this reaction is the opposite of the deamination reaction we talked about in previous slides
recall glutamate and aspartate are very abundant in the body, and these compounds can pass their amino groups to pyruvate, oxaloacetate, etc. to generate the 3, 4, and 5C amino acids
glutamine synthetase is the key enzyme for nitrogen metabolism! —> bc glutamine is one of the major amino sources for transamination (contains 2 amino groups), the enzyme that transfers these amino groups to other compounds to form other amino acids is glutamine synthetase
glutamine synthetase is subject to control via covalent modification
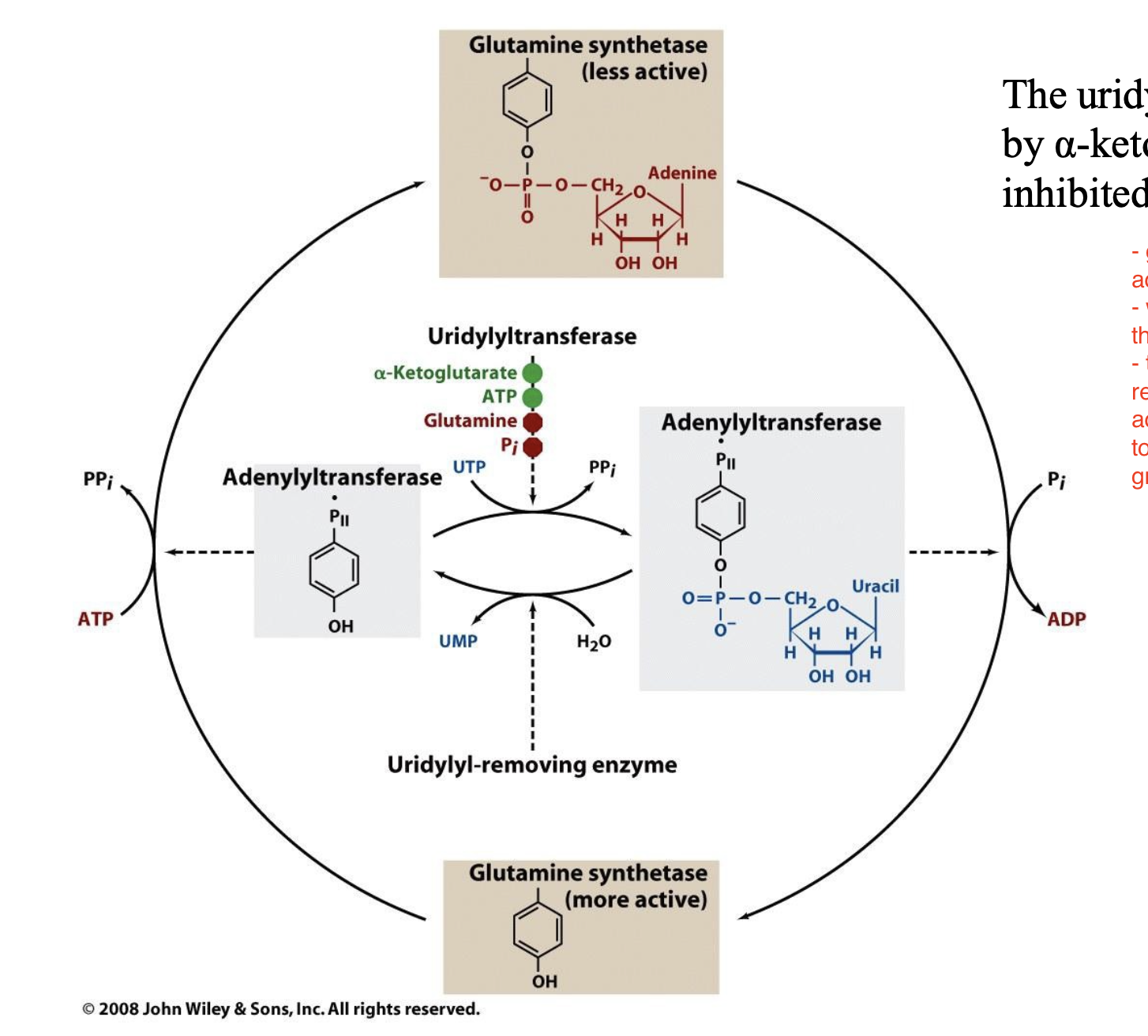
E. coli glutamine synthetase is covalently modified by ______________. The uridylyltransferase is activated by _____________ and inhibited by ___________.
adenylylation (addition of an AMP group) of a specific Tyr residue; α-ketoglutarate and ATP; glutamine and Pi
Explain the covalent modification of glutamine synthetase
glutamine synthetase is covalently modified by adenylylation (as opposed to phosphorylation)
we add an AMP group (bulky group) to deactivate the enzyme
Adenylyltransferase is regulated by binding to a regulatory protein called PII
PII can be phosphorylated or dephosphorylated.
Phosphorylated PII promotes deadenylylation (activating glutamine synthetase)
Unphosphorylated PII promotes adenylylation (inhibiting glutamine synthetase)
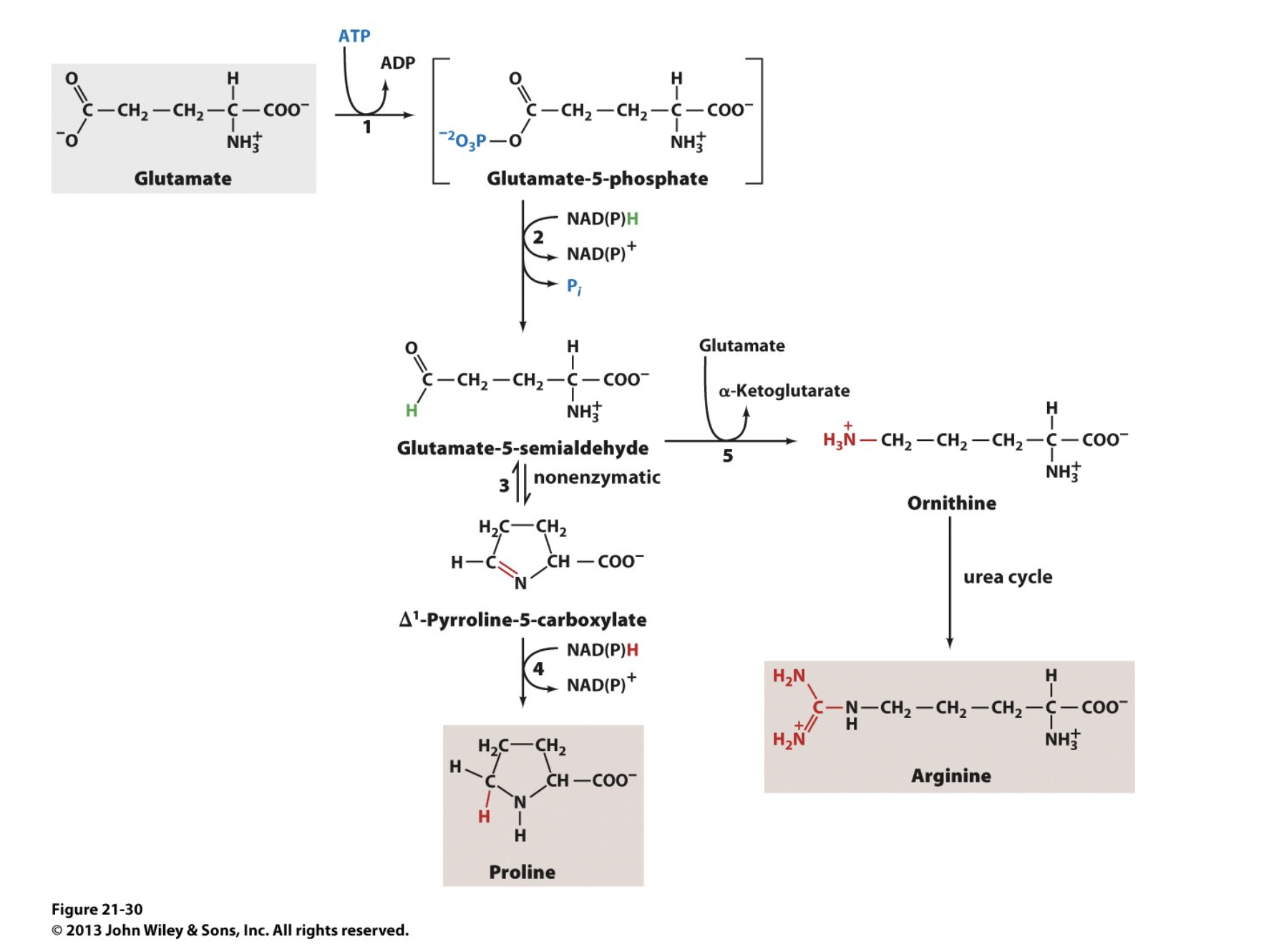
Glutamate is the Precursor of Proline, Ornithine, and Arginine
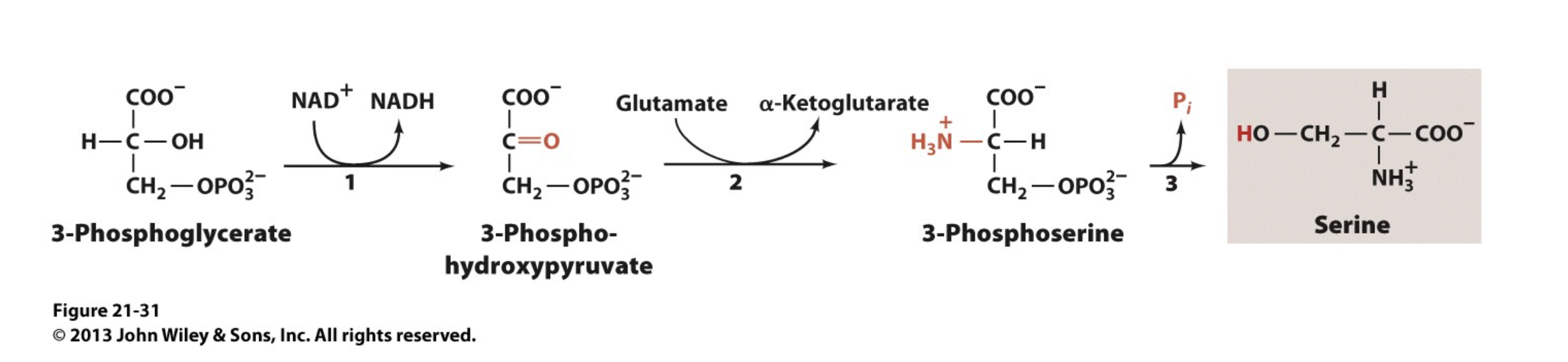
Serine, Cysteine, and Glycine are Derived from 3-Phosphoglycerate
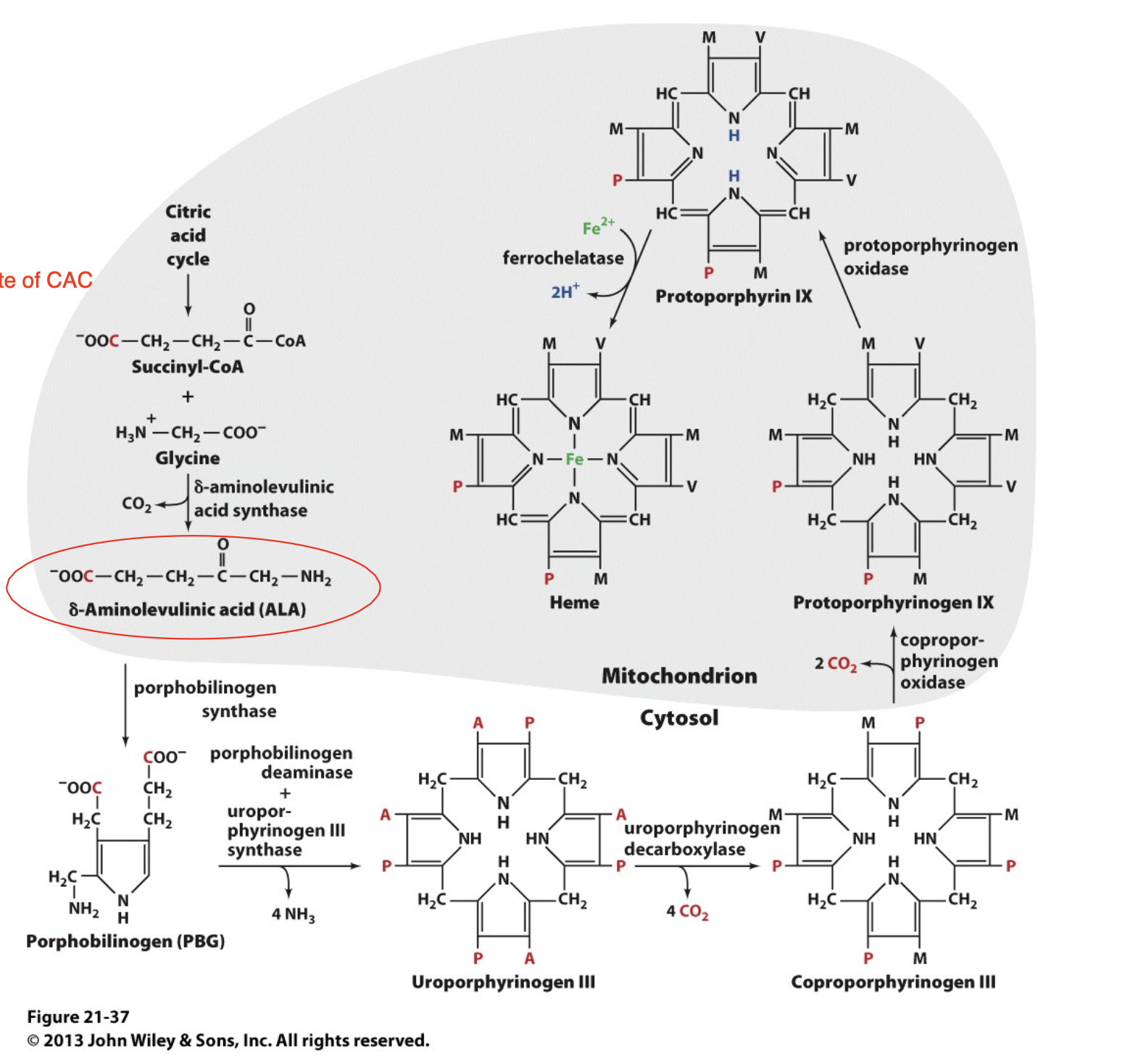
Heme is synthesized from Glycine and Succinyl-CoA
glycine is an amino acid
succinyl-CoA is an intermediate of the CAC
we can synthesize the Heme group by combining glycine and succinyl CoA