5 & 6. Human Genome Editing and Ethics of Human Genome Editing
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
CRISPR/Cas 9
What does CRISPR stand for?
What does Cas stand for?
What is CRISPR/Cas9?
Will CRISPR continue into the future?
CRISPR = clustered regularly interspaced short palindromic repeats.
Cas stands for CRISPR-associated protein.
CRISPR/Cas9 is a powerful gene-editing technology that allows scientists to precisely alter DNA sequences in living organisms, making targeted modifications.
The CRISPR revolution is considered to be far from over, with significant future advancements expected.

CRISPR/Cas 9 mechanism
What does Cas9 do?
How is sequence specificity determined?
What does the host cell then do after Cas9?
Which 2 ways can the break be repaired?
What is the result if a repair template is provided?
What is the issue of the cell choosing the repair pathway?
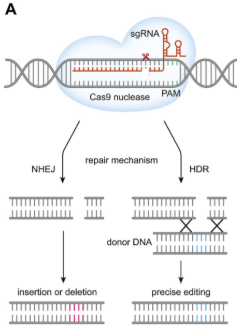
The Cas9 nuclease creates a double-strand break at a unique site in the genome using its 2 nuclease domains which each make a nick.
The sequence specificity is determined by the synthetic guide RNA (sgRNA). The gRNA directs Cas9 to a specific DNA target.
The host cell repairs the break.
Non-Homologous End Joining (NHEJ) which is error prone and could knock the gene out. The other option is homology directed repair (HDR) where you provide the cell with a portion of DNA that’s homologous to the sides before and after the break point (repair template).
If a repair template is provided, the break is frequently repaired by HDR, allowing for gene replacement.
You cannot choose the repair used, you’re hoping that the cell uses your repair template. The problem with diploid organisms is that you already have another copy (your other parent’s). So you can’t control it to repair in your way every time. In an in vivo situation you’re likely to see both HDR and NHEJ and variations within them due to the number of cells.
What uses can CRISPR have (4)?
What is the original main use?
What can it be used as a tool for with genes?
What use can it have when Cas9 is altered?
What other use is being worked on if Cas9 is altered?
Cutting DNA at a specifically targeted location - using Cas9 and a guide RNA (gRNA).
A great tool for knocking out specific genes - The repair process of the double strand break is error prone and often inadvertently introduces mutations that disable the gene.
Making double strand breaks isn’t all that CRISPR can do:
Precise gene editing - Some researchers are deactivating one or both of Cas9 cutting domains and fusing new enzymes onto the protein. Cas9 can then be used to transport those enzymes to a specific DNA sequence.
This could be used to turn a disease causing mutation into a healthy version of the gene.
Not all about gene editing
Labs working on promoting gene transcription - Deactivating Cas9 (can no longer cut DNA), and then attaching transcriptional activators, which recruit the cell’s transcription machinery. This can increase the transcription of the gene.
Gene therapy for metachromatic leukodystrophy (MLD)
What is MLD caused by & what does this affect?
What chemical builds up & what impact does this have?
What does this consequently do to children?
Caused by a mutation in the gene ARSA that means children cannot produce an enzyme which is important for metabolism.
A chemical called sulfatides build up which gradually destroy the protective layer around cells - particularly in the brain where these fatty sheaths are really needed. This leads to the deterioration of anything that requires your brain or a nerve.
So this leads children to gradually lose the ability to walk, talk, eat, see, hear and then breathe.
Specific MLD case study
Who received this gene therapy treatment?
Why did her sister not also receive treatment for her MLD?
Where does the genetic modification occur & what does this allow?
What was the process for the gene therapy?
What result did the gene therapy produce?
Would this treatment work for other kids too?
A 19-month-old baby girl called Teddi was the first child in the UK to receive a life-saving gene therapy treatment for the fatal disorder, metachromatic leukodystrophy (MLD).
Teddi and her older sister were diagnosed at the same time but her sister was not eligible for treatment as the therapy requires the gene treatment to be administered before the irreversible damage caused by the disease progresses too far.
Modification happens outside the body so you can check for the change you want before you pump it back in
Extracted Teddi’s stem cells from her blood and used a viral vector that replaced the mutated copy of the gene with a functioning copy of the ARSA gene. The modified cells were checked to ensure they are functioning correctly before returning the cells to Teddi.
Resulting in her body being able to make the cells with the appropriate enzyme which will reduce the amount of sulfatides in her body.
This treatment only worked for her - her specific mutation and her specific cells. It would have to done again for a different child with the same disease.
CRISPR babies
What was the name of the Chinese scientist who claimed to have made CRISPR babies?
When was it announced and how?
What example of genetic editing is this?
What gene was edited & what disease is it related to?
What did he attempt to do to the gene?
What are the 4 problems with this scientist’s experiment?
What was the legal consequence of this experiment for the scientist?
He Jiankui
Announced in 2018 based on the information he told journalists because it has not been published.
An example of germline editing.
CCR5 gene which is a cell surface protein that some strains of HIV use to get into cells. It was already known that some people who have a natural variant of CCR5 are more resistant to HIV infection. It was thought that HIV struggles to bind to and get into cells. But not resistant to all strains, just some of them.
So he attempted to reproduce this mutation which was a 32 base pair deletion so that offspring will be resistant to getting HIV. All couples with a HIV positive father and HIV negative mother. Hoping to mimic the natural resistance in humans.
Problems:
No evidence that he ever got the 32 base pair deletion correct - they saw different numbers of deletion (since you can’t control the repair process).
He needed to mutate both copies - it’s no good having one copy that the HIV doesn’t bind to but the other is working normally - as it wouldn’t reduce transmission rate - no evidence he got both copies.
He didn’t get any ethical approval from anybody (incl. parents, governments, e.t.c).
No evidence he did a good job of checking for off-targets - CCR5 is from a very large family of genes which encode 7 transmembrane proteins - making it likely similar to hundreds of other G-coupled receptors. No checks were done to see if only CCR5 was mutated.
He was found guilty of conducting “illegal medical practices” and sentenced to 3 years in prison.
Potential Applications of Heritable Genome Editing
What is heritable genome editing?
What are the categories of potential applications of Heritable Genome Editing?
What is category A?
What is category B?
What is the difference between cat. A & B?
What is category C?
What is category D?
What is category E?
What is category F?
Heritable genome editing: any changes that will be inherited to that individual’s offspring. It’s really important if you note whether a change is heritable or not because it really impacts on the ethics.
Categories A - F
Category A - Cases of serious monogenic diseases which ALL children would inherit the disease genotype (e.g. Huntington’s disease (v rare))
Category B - Serous monogenic diseases with some not not all of a couple’s children would inherit the disease-causing genotype (e.g. Cystic fibrosis)
The difference between cat. A & B is the likeliness of getting a disease, it is nothing to do with the seriousness.
Category C - Other diseases with less serious impact than those in A or B (familial hypercholesterolemia)
Category D - Polygenic diseases (Type II diabetes/ schizophrenia/ some cancers) - many genes would need to be changed to reduce your risk.
Category E - Other applications (not heritable diseases) (e.g. EPO gene for endurance sports, thousands of genes linked to intelligence)
Category F - Genetic conditions that result in infertility - considered separately.
Casgevy - Case Study of example of heritable gene editing therapy available now.
What gene is Casgevy therapy used on & what does it do?
What diseases is it used to treat (2)?
What ages can it treat?
It was the first ___________ approved for use in the UK.
Describe these diseases.
Name the pharmaceutical provider.
What does the therapy do?
What can the therapy also do for one of the diseases?
BCL11A - which regulates production of haemoglobin.
Sickle cell disease, Transfusion-dependent β-Thalassaemia.
Patients 12 years and older.
It was the first CRISPR-based medicine approved for use in the UK.
Inherited blood disorders causing abnormal red blood cells.
Bluebird bio.
Modifies a a patient’s own blood stem cells using CRISPR-Cas9 tech to modify the BCL11A gene in the stem cells. It aims to increase production of foetal haemoglobin (HbF) which has a higher affinity for oxygen than the adult form (HbA).
For Sickle cell disease HbF can also inhibit polymerisation of sickle haemoglobin (HbS), reducing the severity of the disease.
What is the difference between heritable and somatic genome editing?
Heritable Human Genome Editing (HHGE) involves making alterations to the DNA in germline cells (sperm, eggs, e.t.c) or in a zygote (the single cell resulting from fertilisation) or early embryo. Because these cells contribute to the genetic material of future offspring, any changes made through HHGE can be inherited by subsequent generations. HHGE is editing in germline cells done in a clinical context with the intent of transferring any resulting embryos to a woman's uterus for gestation.
Somatic Genome Editing, on the other hand, involves making genetic modifications to somatic cells, which are all the cells in the body except for the germline cells. The effects of genome editing in somatic cells are generally limited to the individual being treated and are not transmissible to their offspring.
What are some examples of applications of genome editing in human health (4)?
(Nuffield genome editing ethical review)
Health research using genome edited animal models to study genetic diseases (including ‘personalised’ disease models).
Treating disease, through cell-based therapies (e.g. treating HIV or leukaemia using genome-edited white blood cells) or gene therapy to correct mutations (e.g. muscular dystrophy).
Avoiding the inheritance of single gene conditions, e.g. cystic fibrosis and thalassaemia’s.
Introducing gene variants that confer ‘desirable’ phenotypic traits.
What are some examples of the key ethical concerns around the application of genome editing in human health (5)?
(Nuffield genome editing ethical review)
Concerns about the risks of unintended effects due to off-target DNA alterations.
Concerns about the implications of genome editing in reproductive treatment, for example, making changes that will be passed on to future generations. Issues including outcomes, risks, costs and societal impact have implications for governance and regulation.
Concerns that widespread of genome editing may amount to ‘liberal’ eugenics driven primarily by the choices of parents.
Concerns that potential benefits and harms of genome editing might not be distributed equitably.
Concerns about how to delineate morally acceptable and unacceptable uses of genome editing for governance purposes.
What are some other examples of applications of genome editing in areas other than human health (3)?
(Nuffield genome editing ethical review)
Improving efficiency of development/production of crops.
Creation of genetically altered insects to control disease/crop pests (e.g altered mosquitos to halt spread of malaria).
Industrial application in bacteria (e.g in fossil fuel alternatives, antibiotics, vaccines).
In the Nuffield genome editing ethical report what potential application of genome editing in humans was stated as an issue that should be addressed urgently based on societal and moral issues?
Genome editing in human reproduction – i.e. intergenerational alterations (e.g. for the purpose of avoiding genetic disease).
Identify stakeholders in genome editing:
Evaluate the risks and benefits of genome editing:
Identify credible sources to support arguments:
Foster responsible engagement with emerging technologies
Stakeholders in hereditary genome editing
Parents, family, the child themselves.
Medical professionals (before and after treatment)
People in pharmaceutical industry that treat the condition
Future generations of offspring
Religious groups/cultures that may believe it to be right or wrong
People currently alive with the condition (e.g DS - how do they feel about it if the disease is being edited out of society)
People in charge of policy making
Benefits of CRISPR technology
Modification of pathological genes (e.g Huntington’s gene)
Ability to “fix” single base changes (could cure a disease in the form of prevention)
Would allow couples with a high risk chance of having a child with significant health challenges to have children
Risks/harms of CRISPR technology
Serious injury/disability and/or death to research participants and/or offspring
Inequitable access and exacerbation existing inequalities (experiments are incredibly expensive because they are personalised to each case/family)
Misapplications and eugenics (when CRISPR is more used/understood)

Which category (A to F) does the CCR5 Δ32 editing (CRISPR babies) fall into?
Category E because HIV is not a heritable disease.
There are alternative effective methods for preventing and treating HIV which are widely available.
Benefit to harm ratio is uncertain in these situations when discussing possible genome editing.
Consensus study report of heritable human genome editing in 2020 by the international commission on the clinical use of human germline genome editing.
Which categories are stated to be not currently suitable for HHGE?
What do they describe category E as?
What is the reasoning behind declaring them not currently suitable for HHGE?
What would future justification for pursuing these interventions require?
As stated in a consensus study report of heritable human genome editing in 2020 by the international commission on the clinical use of human germline genome editing.
Category D and E are not currently suitable for HHGE.
Category E are genetic changes that are not directed toward variants involved in heritable diseases and may involve genetic sequences that do not naturally occur in human populations and uses that could be seen as enhancements.
Scientific understanding and existing technologies are insufficient to produce predictable, well-characterized results, including across a range of genetic and environmental interactions, and to minimize the effects of unknown and speculative risk. Moreover, these uses raise additional societal and ethical concerns.
Any future justification for pursuing such interventions would require scientific agreement that the long term impact of such change can be assessed, and societal approval about the acceptability of such interventions.
Somatic genome editing Case Study
detail
stakeholders
risks/benefits of this application
what would you advise a family member
link to the reports - use words and stuff from them
CTX001 (autologous cellular drug) is the patient’s own cells.
People immediately involved
Others in society who might be indirectly affected and society as a whole
pig kidney example