Module 3: Biochemical Pathology of Diabetes
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
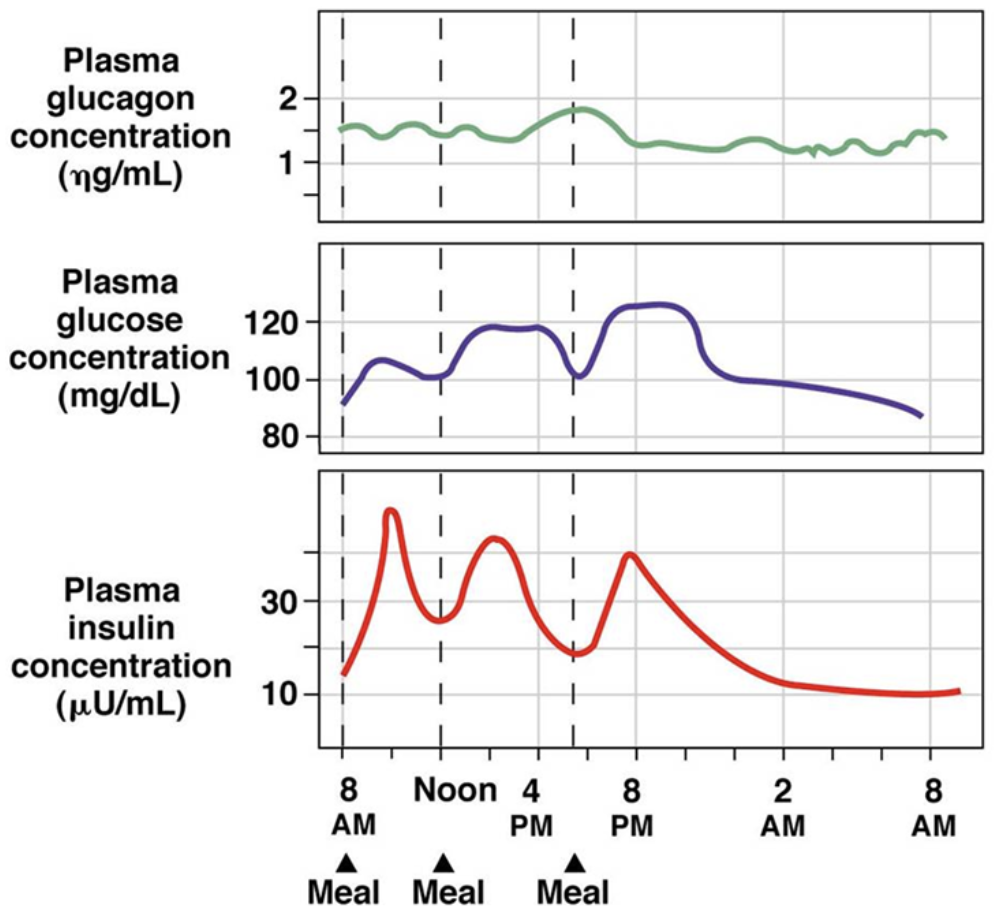
How do your hormone levels change with blood glucose levels? What is the homeostatic blood glucose point?
the homeostatic blood glucose point is 5 mmol/L
as blood glucose rises, insulin concentration is increased as pancreatic beta cells release it → encourages the body to take glucose into cells for energy or storage (glycogen in the liver)
as blood glucose lowers, insulin levels drop while glucagon levels increase as pancreatic alpha cells release it → glucagon encourages the body to release glucose from glycogen stores so that the blood has enough glucose to deliver to the heart and brain
What is diabetes mellitus? What systems can it impact? What are risk factors?
diabetes is a disorder in glucose metabolism characterized by hyperglycemia from insulin secretion impairment, defective insulin action, or both
it can impact both microvascular and macrovascular systems
microvascular: retinopathy, nephropathy, neuropathy
macrovascular: ischemia, stroke, peripheral vascular disease
risk factors include obesity, high blood pressure, age >45, low levels of high-density lipoprotein, high levels of low-density lipoprotein, high levels of triglycerides, low levels of physical activity, or history of heart disease/stroke
What is type 1 diabetes? What are the symptoms and when do they develop? How is it treated?
type 1 diabetes refers to the hyperglycemic condition caused by an autoimmune reaction within the body that prevents it from making insulin → the autoimmune response destroys the islet cells of the pancreas, making it unable to produce insulin
symptoms develop quickly and present in childhood or early adulthood and symptoms of untreated T1 diabetes include
unintended weight loss → body can’t break down carbs without insulin, so it breaks down the body’s fat and muscle
increased thirst, hunger, urination, and irritability
blurred vision, eye and foot damage
individuals with T1D need to take insulin every day to survive
Describe the pathogenesis of type one diabetes. What are the stages?
triggered event(s): something triggers the autoimmune destruction of the pancreatic beta cells in people at genetic risk
stage 1 (normoglycemia): patient is asymptomatic but they have autoantibodies
stage 2 (dysglycemia): patient has an impaired metabolic response to glucose
stage 3 (hyperglycemia): patient has an insulin deficiency, hyperglycemia, and a loss of beta cell function
What is type 2 diabetes? What are the symptoms and when do they develop? How is it treated? What are the two theories of pathogenesis?
type 2 diabetes is a hyperglycemic condition in which the body ineffectively uses insulin to control glucose levels
symptoms develop over many years and it’s typically diagnosed in adults
it can be prevented or delayed with lifestyle changes and it’s treated with antihyperglycemic agents
there are two theories of T2D’s pathogenesis
insulin insufficiency theory: the amount of insulin produced isn’t enough to induce adequate signalling to prevent hyperglycemia
twin cycle hypothesis: excessive eating can result in fatty liver, which results in high fatty acid exposure to the pancreas and makes it dysfunctional
What is the twin cycle hypothesis for? Describe this hypothesis? What treatment has it resulted in?
the twin cycle hypothesis aims to explain the pathogenesis of type 2 diabetes and it consists of two cycles that interact to produce the condition
liver cycle: excessive eating can cause fat build-up in the liver, decreasing its ability to respond to insulin (resistance)
the resistance leads to increases in blood glucose and insulin levels
in early T2D, beta cells respond to the insulin resistance by releasing more insulin which reinforces this cycle
pancreas cycle: the fatty liver exports very low density lipoprotein triglycerides and the exposure to high fatty acid concentrations initially makes the pancreas release more insulin
as the condition goes untreated, the beta cells become dysfunctional and fail to maintain blood glucose levels
this theory revealed that cells are overwhelmed by fat and can’t respond to insulin → introduces a new treatment strategy to reduce the load on cells
drugs that clear glucose through the urine have shown to be succesful
Describe insulin receptor signalling. What dysregulation results in type 2 diabetes?
insulin receptor (INSR) signalling contributes to our maintenance of blood glucose levels → these receptors are tyrosine kinase receptors, so their signalling cascades involve a series of phosphorylations
there are two components of INSR dysregulation
decreased INSR tyrosine kinase activity
decreased surface INSR content
without proper responses to insulin, we see insulin resistance and thus type 2 diabetes develops
What is gestational diabetes? What are the causes? What are complications associated with this condition?
gestational diabetes is a glucose intolerance with the onset or recognition during pregnancy and it occurs because the body can’t make enough insulin during pregnancy
when pregnant, the body produces many hormones and undergoes a lot of changes → these changes cause the body’s cells to use insulin less effectively and results in insulin resistance
complications include
macrosomia (big baby): high glucose levels cause the baby’s pancreas to make more insulin to take in the glucose, which gets converted to fat
hypoglycemia in the baby immediately after birth → the baby is used to high glucose levels, so it produces high insulin levels but the high insulin extends until birth and glucose levels drop
gestational diabetes usually disappears after birth, but it leaves an increased risk of developing type 2 diabetes later in life for the mother and the baby
What is mature onset diabetes of the young? What causes it?
mature onset diabetes of the young (MODY) is a rare diabetes with an onset between 10 - 40
it shared genotypic features with both types of diabetes
it has an autosomal dominant inheritance pattern
it’s caused by mutation in one of three/four genes that result in defects in beta cell insulin secretion
mutations can impair insulin sensing, glucose metabolism, and activation of ATP-dependent potassium channels involved in insulin signalling
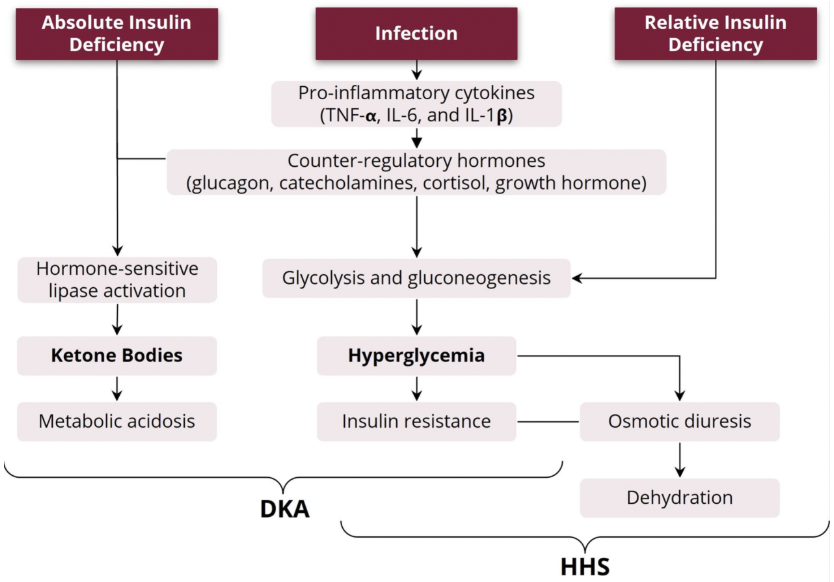
What is decompensated diabetes? What is diabetic ketoacidosis? What are the symptoms? What is hyperglycemic hyperosmolar states? What are the symptoms?
decompensated diabetes refers to diabetes improperly treated such that a hyperglycemic emergency arises
two hyperglycemic emergencies are diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS)
when the body doesn’t make enough insulin to catabolize glucose into usable energy, it begins to undergo ketogenesis → this results in the buildup of acids/ketone bodies, this is diabetic ketoacidosis
it’s more common in young people with type 1 diabetes
symptoms: fast, deep breathing, fruity-smelling breath, tiredness, nausea, and vomiting
hyperglycemia for a long time can lead to severe dehydration, confusion, and hyperglycemic hyperosmolar state
when blood glucose levels aren’t controlled, the excess glucose is released in urine which causes increased urination and dehydration if left untreated
it’s more common in adult and elderly people with type 2 diabetes
symptoms: confusion, extreme thirst, frequent urination, fever, and blurred vision
What is metabolic decompensation? How does it arise in diabetes? How does this differ for diabetic ketoacidosis and hyperglycemic hyperosmolar state?
a compensated system functions despite stressors or defects
metabolic decompensation refers to the breakdown of previously functional metabolic pathways due to many factors (ie. stress, sickness, old age, fatigue, etc.)
decompensated diabetes can cause these hyperglycemic emergencies in many ways
DKA and HHS can develop due to infection of inadequate insulin therapy
inadequate treatment of type 1 diabetes causes absolute insulin deficiency which, along with infection, can cause diabetic ketoacidosis
inadequate treatment of type 2 diabetes causes relative insulin deficiency which, along with infection, can cause hyperglycemic hyperosmolar state
Why do we look at metabolic acidosis when examining metabolic decompensation? How do we determine the extent of metabolic acidosis? How do we do this/what techniques are used?
we evaluate metabolic acidosis to help determine what hyperglycemic emergency a patient may be experiencing → patients with diabetic ketoacidosis will have metabolic acidosis due to ketone bodies whereas patients with hyperglycemic hyperosmolar state will not
testing for serum bicarbonate can help
[HCO3-] below 22 mmol/L is an indicator of metabolic acidosis
we test for serum bicarbonate using a carbonic anhydrase assay
this uses bicarbonate in a series of reactions to convert oxaloacetate to malate → the consumption of NADH indirectly reflects the amount of bicarbonate that was present in the sample
![<ul><li><p>we evaluate metabolic acidosis to help determine what hyperglycemic emergency a patient may be experiencing → patients with diabetic ketoacidosis will have metabolic acidosis due to ketone bodies whereas patients with hyperglycemic hyperosmolar state will not</p></li><li><p>testing for serum bicarbonate can help</p><ul><li><p>[HCO3-] below 22 mmol/L is an indicator of metabolic acidosis</p></li></ul></li><li><p>we test for serum bicarbonate using a <mark data-color="green" style="background-color: green; color: inherit">carbonic anhydrase assay</mark> </p><ul><li><p>this uses bicarbonate in a series of reactions to convert oxaloacetate to malate → the consumption of NADH indirectly reflects the amount of bicarbonate that was present in the sample </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/040b86d5-77b9-4ab1-9e5b-5ac3a9f59142.png)
What is the principle of plasma neutrality? How is it used?
the principle of plasma neutrality states that anions must balance cations to maintain a neutral charge in the blood
calculating the anion gap can reveal the type of metabolic acidosis presenting in a patient — note: need to be mindful of what ions you’re using to measure so that you use the right reference value
What changes in glucose, fatty acids, and ketone bodies are associated with diabetic ketoacidosis?
with absolute insulin deficiency, glucose isn’t properly released and metabolized using the TCA cycle
the body needs energy so it triggers the generation of free fatty acids from triglycerides → these can be beta-oxidized to form acetyl-CoA for the TCA cycle for energy
this leaves the TCA cycle overwhelmed by acetyl-CoA, so excess is converted into ketone bodies in the liver
note: we can determine the amount of ketoacidosis by measuring the levels of ketone bodies in the blood
What are the principle ketone bodies? How are they metabolized? How do these indicate diabetic ketoacidosis?
the principle ketone bodies are beta-hydroxybutyrate (BHB) and acetoacetate (AcAc)
AcAc can be metabolized to acetone non-reversibly or BHB reversibly
with diabetic ketoacidosis, we see an increased BHB:AcAc ratio due to enhanced fatty acid beta oxidation (reliance on fats for energy, but it overwhelms the TCA cycle with acetyl-CoA)
levels of BHB greater than 0.5 mmol/L are considered abnormal
What were nitroprusside test tablets used for? Why are they no longer used/what are their limitations?
nitroprusside test tablets were the first tests for ketone bodies → they can measure blood and/or urine acetone and acetoacetic acid levels, but not BHB levels
limitations
tablets only react to AcAc and, to a small degree, acetone
with diabetic ketoacidosis, beta-hydroxybutyrate (BHB) is the main metabolic product so the severity of the ketosis could be underestimated since it only looks at AcAc
What test is used to evaluate the potential presence of diabetic ketoacidosis? What are the two methods of administering this test?
beta-hydroxybutyrate tests are used to measure BHB levels in the blood, serum, or plasma
levels of BHB greater than 0.5 mmol/L are considered abnormal and elevated levels may require treatment for DKA
bedside ketone meters can be used to initially diagnose DKA → they’re a point of care test that measures serum BHB levels with a reagent strip
laboratory testing of serum or capillary BHB levels can be used as a follow up to confirm a diagnosis, analyze the effectiveness of treatment, or evaluate suspected alcoholic ketoacidosis
What is glycemic control? How does it differ in people with type 1 vs type 2 diabetes?
glycemic control describes a person’s physiological response to carbohydrate load over a period of time
a person with type 1 diabetes has poor glycemic control without the administration of insulin because they can’t effectively respond to a glucose load
a person with type 2 diabetes has blunted glycemic control, but this can be modified with antihyperglycemic agents
What are the four ways in which we can measure glycemic control?
random plasma glucose test: glucose is measured from a sample taken at any time, without regard to the timing of the last meal → allows clinicians to see the amount of glucose in a person’s blood at a particular time
fasting plasma glucose test: glucose is measured from a sample taken after the person hasn’t eaten in 4-8 hours
glycated hemoglobin (HbA1c) test: the average amount of glucose in an individual’s blood is measured over the past 90 days → can be used to evaluate the effectiveness of glycemic treatment
oral glucose tolerance test: glucose is measured from a sample taken in a fasted state and then again after the patient drinks a cup of sugary liquid → allows clinicians to monitor the body’s ability to use glucose
How is type 1 diabetes diagnosed? What tests are involved?
symptoms develop rapidly, so a diagnosis can be made in the emergency room → a POC glucose test could be conducted, but it alone isn’t sufficient for diagnosis
random blood glucose, fasting glucose, and HbA1c tests can be used to diagnose T1D
it can also be determined using symptoms in addition to other lab tests (ie. tests for autoantibodies)
How is type 2 diabetes diagnosed? What tests are involved? Who is recommended for screening?
diagnosis occurs through random blood glucose tests, fasting glucose tests, and /or HbA1c tests
it can be differentiated from T1D with clinical signs/symptoms
screening is recommended with diagnostic tests for high risk groups such as
people under 45 who are overweight with one or more risk factors
women with a history of gestational diabetes
individuals diagnosed with prediabetes
overweight children with a family history of T2D
How is gestational diabetes diagnosed?
between 24 - 28 weeks of pregnancy, tests for gestational diabetes occurs
clinicians use blood tests for diagnosis, like the oral glucose tolerance test → high glucose levels at any two or more test times following the tolerance test means the patient has gestational diabetes
What are the reference values for each test used to diagnose diabetes?
the random plasma glucose must be greater or equal to 11.1 mmol/L for diabetes to be diagnosed
the fasting plasma glucose level must equal or exceed 7 mmol/L for a diabetes diagnosis
for a glycated hemoglobin test, HbA1c must equal or exceed 6.5% for diagnosis
in the 75g oral glucose tolerance test, two hour plasma glucose must be greater or equal to 11.1 mmol/L for diagnosis
What are the two enzymatic methods for measuring glucose levels in the blood? Describe each approach.
glucose oxidase method: glucose oxidase converts glucose, water, and oxygen to gluconic acid and peroxide → the peroxide reacts with colourless, reduced O-dianisidine to produce brown, oxidized O-dianisidine
the colour change and absorbance can be monitored and used to evaluate glucose levels
endogenous substances, like uric acid and hemoglobin, may cause falsely low glucose results in this method
hexokinase method: hexokinase converts glucose to glucose-6-phosphate, which is turned to 6-phosphogluconate with the reduction of NADP+ to NADPH
the absorbance is monitored to detect changed in NADPH concentration, which reflects the amount of glucose in the sample
What are some pre-analytical variables that can influence glucose test results?
sample temperature impacts analyte metabolism → at room temperature, red blood cells continue metabolism so glucose concentration decreases at a rate of 10% per hour
there are interferences associated with both hexokinase and glucose oxidase methods
the type of blood tube used can influence analyte stability → tubes containing fluoride and oxalate minimize the metabolism of glucose
What is hemoglobin? What does it do? What are the forms/composition? What is HbA1c?
hemoglobin the the red-pigmented, iron-containing protein located in red blood cells → it transported oxygen and carbon dioxide in the blood
it consists of different forms (adult and fetal) and derivatives (acetylated and glycated)
adult hemoglobin makes up over 95% of hemoglobin and consists of four protein chains: two alpha and two beta chains
HbA1c is a glycated hemoglobin that is formed when various sugars attach to the HbA molecule
How is HbA1c formed? What are the steps? What is it used for?
HbA1c is formed in two non-enzymatic reactions that combine glucose with the N-terminal amino group of HbA’s beta chain
the first step is reversible and yields labile HbA1c (Schiff base)
the amount of HbA converted to HbA1c increases with the concentration of glucose
the labile fraction/Schiff base changes rapidly with acute changes in blood glucose concentration → HbA1c shows changes that don’t reflect long time-averaged glucose concentrations
the labile fraction is 5-8% in healthy and 8-30% in diabetic people
the Schiff base is rearranged to form stable HbA1c through Amadori rearrangement
red blood cells live 120 days and HbA1c forms gradually throughout this period
the Amadori rearrangement requires a constant erythrocyte lifespan, free permeability to glucose, and nonenzymatic glycation directly proportional to the glucose concentration → when these conditions are met, HbA1c reflects the average blood glucose levels over the previous 120 days in normal patients
stable HbA1c is suitable to monitor long-term blood glucose control in individuals with diabetes
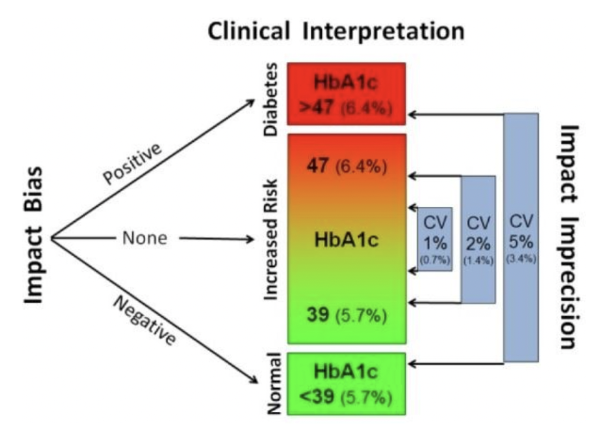
Why does HbA1c need to be standardized? What are the opposing views of the national glycohemoglobin standardization program and the international federation of clinical chemistry?
without standardization, there are large differences between HbA1c results → this could lead to missed or overdiagnosis of diabetes, which could mean erroneous categorization
the National Glycohemoglobin Standardization Program (NGSP) certifies manufacturers of HbA1c instrumentation and manages proficiency testing requirements to reduce variability of results
their testing methods report with the units of % HbA1c
the International Federation of Clinical Chemistry (IFCC) established protocols to standardize HbA1c measures with mass spectrometry and capillary electrophoresis
their testing methods report with the units of mmol/mol (this is what is currently used as the standard)
What is bias in testing methods? What is imprecision? How do our categorization/diagnosing abilities change with imprecision?
bias: the systematic error in the measurement
when due to calibration errors, measurement results that are systematically too high or too low are called positive or negative bias (respectively)
imprecision: the random error in the measurement determined by the reproducibility of the test → this is expressed as the coefficient of variation (the higher the CV, the wider range of results that are at random too high/low points)
as the coefficient of variation increases, the ability to consistently categorize a patient is reduced (ie. a 5% CV means that a patient can flip between diabetic and non-diabetic categorization)
What is glomerular filtration rate? What is clearance rate? How are the two connected?
glomerular filtration rate (GFR) is the measurement of volume filtered through the glomerular capillaries and into the Bowman’s capsule per unit of time → represents the best overall assessment of kidney function at a given point in time
clearance rate is the rate a substance is removed from the body by the kidneys
clearance rate for a substance is equal to the glomerular filtration rate when the substance is neither secreted nor reabsorbed by the kidneys
What is the gold standard technique for measuring glomerular filtration rate? Why isn’t this used? What is currently used?
the gold standard technique for measuring GFR is inulin clearance, which involves IV administration of inulin
this is labour-intensive and expensive for routine clinical use
creatinine clearance is used instead → GFR is measured by combining serum creatinine and creatinine clearance levels
serum creatinine is the most commonly used biomarker for kidney function
What is creatinine? Where is it stored? Why is it a good candidate for analyzing kidney function?
creatinine is a non-protein nitrogenous substance derived from muscle creatine → circulating levels vary with muscle mass and dietary intake of creatine
both creatine and creatinine are stored in the muscles
in the kidneys, creatinine is freely filtered through the glomeruli, not reabsorbed in the tubules, and excreted in the urine
this makes it a good way to analyze kidney function
What are factors influencing creatinine clearance? What are factors influencing serum creatinine levels?
both serum creatinine and creatinine clearance are used for glomerular filtration rate estimations, so it’s important to know influencing factors since these help us analyze kidney function
factors influencing creatinine clearance:
sex: females have less muscle mass and lower rate of creatinine production than males
race: Latin Americans produce lower clearance values and African Americans produce higher values
patients with unique diets or have muscle wasting can produce deviations in creatinine levels
factors influencing serum creatinine:
drugs can increase serum creatinine levels
age, sex, ethnicity, dietary protein intake, and lean mass
it may remain within the reference range despite marked renal impairment in patients with low muscle mass
reference GFR values decrease as age increases, so sensitivity for early detection is poor and isn’t a good predictor when analyzing older adults
What is glomerular filtration rate? How is it calculated? What is the reference value? What do differing values indicate?
glomerular filtration rate is the measurement of volume filtered through the glomerular capillaries and into the Bowman’s capsule per unit of time → many substances can be used to calculate, but creatinine is often used with age and sex as reference intervals
GFR = UV/P
U = urine concentration of a substance (creatinine) in mg/mL
V = urine flow or volume of urine per set time in mL/min
P = plasma concentration of a substance (creatinine) in mg/mL
the normal GFR is 90 mL/min or higher
a GFR below 60 mL/min indicates abnormal kidney functioning
once GFR drops below 15 mL/min, a patient is at high risk for needing kidney failure treatment (ie. dialysis or transplant)
What are the limitations of calculating glomerular filtration rate?
urine volume is difficult to accurately measure and patients often don’t collect all their urine for a 24 hour period
the urine volume is necessary for clearance calculation, so it affects the accuracy of the creatinine clearance (and thus how we evaluate kidney functioning)
this is why GFR is calculated from serum creatinine alone in most scenarios → it’s shown to be more accurate in most settings
What is eGFR? What are the different equations/what do they consider? Which is used today?
eGFR assesses steady kidney function and is based on serum creatinine levels alone (doesn’t use urine levels like GFR)
note: if someone’s kidney function is rapidly changing, eGFR shouldn’t be used
the Cockcoft-Gault equation uses patient height, weight, and/or age to calculate creatinine clearance but these metrics aren’t always available to the lab → imprecise in some situations, but still used for drug dose calculations
the Modification of Diet in Renal Disease (MDRD) equation looks at age, sex, routine analytes, and metabolic profiles → it used to be the most accurate for eGFR but was limited in its ability to determine GFR at higher values (>90)
the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation takes many factors into account and is the most accurate → it’s most current and widely accepted today (but expensive and lengthy)
What is the Jaffe reaction? What is this method used for?
the Jaffe reaction looks at how creatinine and picric acid react in alkaline solutions → the colour change is directly proportional to the creatinine concentration
note: several other compounds induce similar reactions!! not specific!!
the Jaffe method is used for creatinine testing of blood and urine due to its speed, adaptability in automated analysis, and cost effectiveness
What are the limitations of the Jaffe method? What are alternatives for assessing creatinine levels?
the Jaffe method is subject to bias due to interfering substances, so it isn’t as specific
the higher bias at lower creatinine concentrations may lead to an overestimation of GFR, especially in kids, elderlies, and during pregnancy — can lead to patients being seen as healthy even if they’re not
an alternative is to use enzymes to determine creatinine levels → this is more expensive though
What is the Creatinine Standardization Program? What are the four steps?
the Creatinine Standardization Program was created to reduce interlaboratory variation in creatinine assay calibration and to enable more accurate eGFR → this allows for results from different labs to be compared
there are four steps of this program
reference method: a reference method of isotope dilution mass spectrometry was developed so that all creatinine assay manufacturers can compare their results to it
compare to reference: manufacturers can use math to see how their assay results differ from the IDMS result → the equation changes the assay results to one that is comparable to the IDMS result
validate: the equation is validated and the assay becomes “IDMS traceable”
monitor: this is monitored on an ongoing basis to ensure that any variation in assay manufacturing doesn’t influence the accuracy of results
What is albumin? What is albuminuria? How can it be used to evaluate kidney function?
albumin is a protein found in the blood → a healthy kidney doesn’t let it pass from the blood into the urine, so albumin in the urine indicates damage
albuminuria means that you have too much albumin in your urine → it’s a sign of kidney disease
healthcare providers use the ratio of albumin and creatinine measurements to estimate the amount of albumin excreted in 24 hours → a ratio over 30 mg/g is higher than normal and indicates that the protein is in the urine so the kidney is damaged