Acid Base Student
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
What is acid base balance?
Regulating pH, bicarbonate concentration, and partial pressure of carbon dioxide of body fluids
How does cognition affect acid-base balance?
How does fluid and electrolytes affect acid-base balance?
Closely related; changes in one of them = change in another (when we start to see patients with acidosis = they also have hyperkalemia); and vice versa
How does acid-base balance affect perfusion?
How does gas exchange affect acid-base balance?
Retaining CO2 = harder to breathe out = disruptions in various areas
How does acid-base balance affect elimination?
How does nutrition affect acid-base balance?
Normal acid production, whereas starvation or high fat low card diets will disrupt the process (balance)
What maintains pH balance in the body in relation to hydrogen ions?
A steady balance is maintained between acids produced in the body and bases that neutralize and promote acid excretion (in order for cellular environment to thrive)
This balance is essential for regulating hydrogen ion concentration (part of metabolism), which directly influences pH levels in the body
They do this in a small range continuously making small adjustments
What are acids and how do they affect hydrogen ions in body fluids?
Acids are substances that release hydrogen ions (H⁺) when dissolved in water or body fluids
This increases the concentration of free hydrogen ions in the solution
Acids are also produced as end products of metabolism
Ways of acid production: cellular metabolism
Outcomes = Carbonic acid (carbon dioxide and water) & Metabolic acids (Lactic acid)
Acid intake (substances that will convert to acid)
What are bases and how do they affect hydrogen ions in body fluids?
Bases are substances that bind free hydrogen ions (H⁺) in solution, thereby lowering the amount of free hydrogen ions in the solution
Bicarbonate (kidney is responsible for production and retention)
(neutralizes acids)
How do hydrogen ions affect acidity and alkalinity, and how is their concentration expressed?
Acidity or alkalinity depends on the concentration of hydrogen ions (H⁺).
An increase in H⁺ leads to acidity, while a decrease in H⁺ leads to alkalinity.
Hydrogen ion concentration is expressed as pH
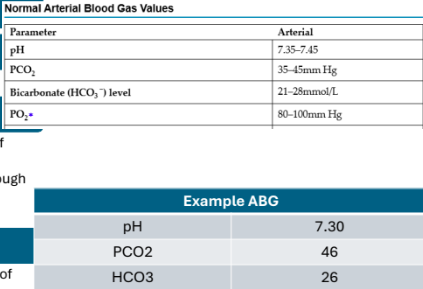
What is the normal range of arterial pH?
7.35 to 7.45
If pH is below 7.35, what state is the client in?
Acidotic (tons of H)
If pH is above 7.45, what state is the client in?
Alkalotic (very little amount of H)
What are the regulatory mechanisms?
Buffer systems: reacts immediately
Respiratory system: within minutes
Renal system: several days
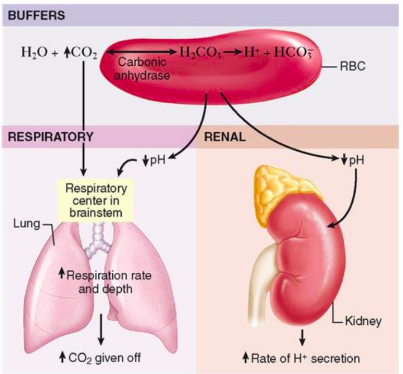
What is the function of the buffer system in the body?
The buffer system is the fastest acting response to changes in pH (primary regulators)
It chemically changes strong acids into weaker acids or binds acids to neutralize their effect, helping maintain pH balance
Carbonic acid-bicarbonate
Phosphate
Protein
Hemoglobin - shifts chloride in and out of RBC in exchange of bicarbonate
Potassium
Cell can also act as a buffer (shifting of in and out of hydrogen) → exchange it with potassium
Kick potassium out; bring hydrogen in
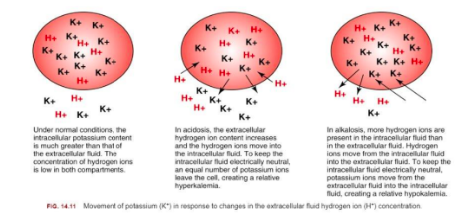
What is the role of the carbonic acid-bicarbonate buffer in the body?
The carbonic acid-bicarbonate system is the major buffer in the body
It helps maintain the pH of blood by balancing carbonic acid (H2CO3) and bicarbonate (HCO3-)
CO2 + water = bicarbonate acid + H + carbonate
Depends on what our body needs; can have CO2 being removed by breathing or have H and carbonate to get removed by renal system
Bicarbonate regulated by kidney: hydrogen removed through urine
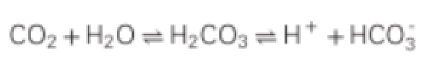
How is potassium a buffer?
Cells can shift H In & Out
Potassium exchanges
Alkalotic: Hypokalemia
ECF has too little H, cell will give its own H (goes out of cell) potassium goes into cell
Acidosis: Hyperkalemia
Cell is trying to neutralize hydrogen (by bringing some in) while kicking potassium out
How does the respiratory system help regulate pH?
The respiratory system provides a fairly quick response (minutes) to pH changes
Cellular metabolism releases CO2, which enters red blood cells (RBCs)
will take CO2 to RBC => transport to lungs to be breathe out
In RBCs, CO2 combines with water to form carbonic acid (H2CO3)
Carbonic acid dissociates into H+ (hydrogen ions) and HCO3- (bicarbonate) (if bicarbonate needs to be removed, gets sent to kidneys if environment is alkalotic)
The H+ is buffered by hemoglobin, while bicarbonate diffuses into the plasma, helping to maintain pH balance
CO2 directly relates to carbonic acid and afterward, H

Where is the respiratory center that detects CO2 or H+ levels located?
The respiratory center (chemoreceptors) is located in the brainstem
How do respirations impact pH levels?
Respirations can remove or retain CO2, which impacts the pH level. (This is only effective if the patient does not have a respiratory condition.)
How does the renal system help regulate pH?
Slower response (days)
Reabsorb and conserve bicarbonate
Generates additional carbonate and eliminate excess H when acidosis
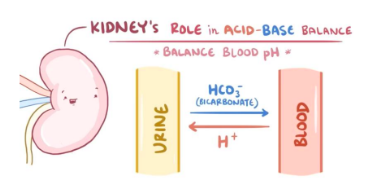
What are the 3 mechanisms of acid elimination (renal)?
Secretion of small amounts of H into renal tubule
Combination of H with ammonia to form ammonium
Excretion of weak acids (urine is acidic)
Reabsorbing, saving bicarbonate (takes hydrogen out of urine system)
What are the alterations in acid-base balance?
Neutral pH: 7.35 - 7.45
Normal ratio: 1 acid to 20 bases
Imbalance: Leads to acidosis (excess acid) or alkalosis (excess base)
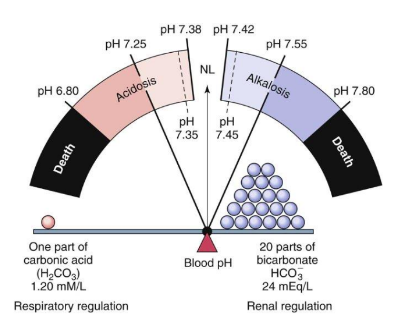
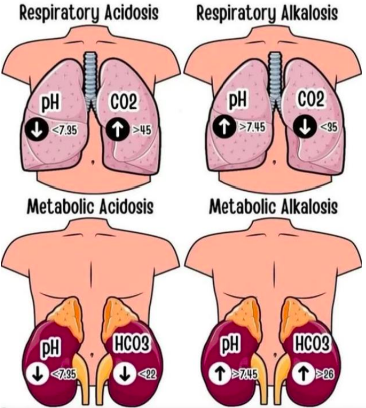
What are the types of imbalances?
Acidosis:
Respiratory
Metabolic
Alkalosis:
Respiratory
Metabolic
What does respiratory acidosis mean?
Carbonic acid excess → CO2 and water
What is the main reason that someone goes into respiratory acidosis?
Hypoventilation is occurring
Respiratory system is impaired leading to retain a lot of CO2 → hypercapnia and acidotic result (due to CO2 turning into carbonic acid)
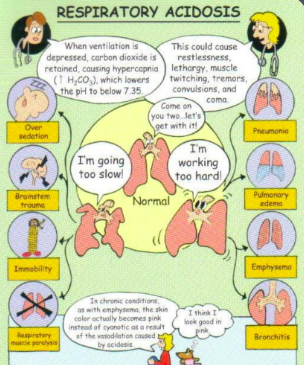
What are the main causes of respiratory acidosis?
Common cause: COPD/severe respiratory conditions (CO2 retainers) → will likely to breathe 30-40 but will still show respiratory acidosis bc of impaired alveoli (air trapping), narcotics (respiratory depression), obesity (not getting all air out → increase hypercapnic levels), severe pneumonia, ateltectasis, respiratory muscle weakness
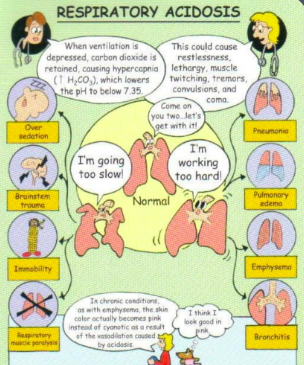
How can we compensate for respiratory acidosis?
Kidneys save bicarbonate back into blood stream, and will promote removal of H+ (into urine) and produce more ammonia
Kussmaul's respirations (deep, rapid breathing)
Treat underlying cause (COPD, pneumonia)
→ bc lungs are not working as they should
What is metabolic acidosis?
More complex; can be too much acid build up in body or too little bicarb in body (goes into acidotic state)
What are the causes of respiratory acidosis?
Common causes:
Too much acid (Diabetic ketoacidosis and increased lactic acid build up, starvation);
Too little bicarbonate (severe diarrhea, renal disease unable to reabsorb bicarbonate)
How can we compensate for metabolic acidosis?
Increase CO2 excretion by the lungs and kidneys (if healthy) reabsorb bicarbonate into blood stream and attempt to remove additional acid
Kussmaul's respirations (deep, rapid breathing) body is compensating for hyprecapnia
What are the clinical manifestations of acidosis (regardless whether its respiratory or metabolic)?
Low pH; acidotic = reduce ability of excitable membranes that respond to cardiovascular tissues, neurons, skeletal muscle, etc.
SLOW
What are the clinical manifestations of acidosis (regardless whether its respiratory or metabolic): cardiovascular?
Hypotension - acidosis caused
Dysrhythmia - Potassium caused by hyperkalemia
Warm flushed skin (peripheral vasodilation)
relaxation of vascular system
What are the clinical manifestations of acidosis (regardless whether its respiratory or metabolic): neurological?
Drowsiness
Disorientation
Dizziness
Headache
Coma
What are the clinical manifestations of acidosis (regardless whether its respiratory or metabolic): respiratory?
Hypoventilation with hypoxia (Respiratory - lungs unable to compensate), pnuemonia, narcotics, COPD
Deep, rapid respirations (Metabolic - compensation), deep rapid respirations = compensate
What are the clinical manifestations of acidosis (regardless whether its respiratory or metabolic): neuromuscular?
Seizures (respiratory only)
What are the clinical manifestations of acidosis (regardless whether its respiratory or metabolic): gastrointestinal?
N/V, diarrhea, abdominal pain (metabolic only)
What is respiratory alkalosis?
Lungs → alkalotic (caused by hypoventilation)
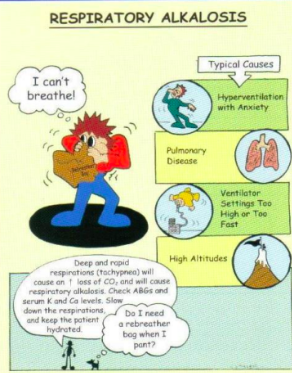
What is the main reason that someone goes into respiratory alkalosis?
Occurs with hyperventilation
Hypoxemia from acute pulmonary disorders (could be disease)
(pneumonia, pulmonary embolus) → too much CO2 out; breathing rly fast
(acute) Pain, anxiety, CNS disorders
Exercise, fever (increased metabolic demands)
How can we compensate for respiratory alkalosis?
Pain & Anxiety- If healthy lungs, rebreathe in a
paper bag
Otherwise, removal of excess bicarbonate (done by kidneys)
What is metabolic alkalosis?
Not a respiratory issue; could be low acid (lactic, hydrochloric) or too much bicarbonate
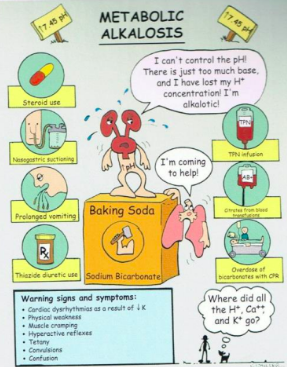
What is the main reason that someone goes into respiratory acidosis?
Low acid or high bicarbonate
Causes:
Low acid: prolonged vomiting or gastric suction = in stomach, if pt is vomiting, getting rid of acid = alkalotic state
High bicarb: ingestion of baking soda, diuretic therapy, excessive steroids
How can we compensate for metabollic alkalosis?
Respiratory rate decreases to retain CO2
Not a lung issue so lungs compensates right away (hypoventilate)
Renal removal of bicarbonate
What are the clinical manifestations of alkalosis (regardless whether its respiratory or metabolic)?
Decreased excitable tissues
What are the clinical manifestations of alkalosis (regardless whether its respiratory or metabolic): neurological?
Light headedness
Confusion
What are the clinical manifestations of alkalosis (regardless whether its respiratory or metabolic): cardiovascular?
Tachycardia
Dysrhythmia - potassium = hypokalemia
What are the clinical manifestations of alkalosis (regardless whether its respiratory or metabolic): gastrointesinal?
N/V
What are the clinical manifestations of alkalosis (regardless whether its respiratory or metabolic): neuromuscular?
Tetany = spasms/tightening of muscles
Tingling of extremities
Hyperreflexia/hypertonic muscles/cramps
Excitable signs based on an alkalotic environment
What are the clinical manifestations of alkalosis (regardless whether its respiratory or metabolic): respiratory?
Hyperventilation (respiratory - lungs unable to compensate)
Hypoventilation (metabolic – compensation)
What is a way we can diagnose acid base?
Arterial Blood Gas (acutely ill/complex)
Acid base status
Origin of imbalance - if CO2 is acidotic range, pH is acidotic = respiratory/bicarbonate = acidotic, pH is acidotic = metabollc
Idea of body’s ability to regulate pH
Overall oxygen status
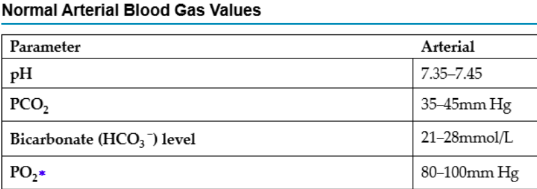
What does pH refer to?
Balance of hydrogen ions in the arterial blood. A high pH indicates a solution is alkaline (low H+ concentration) and a low pH indicates a solution is acidic (high H+ concentration)
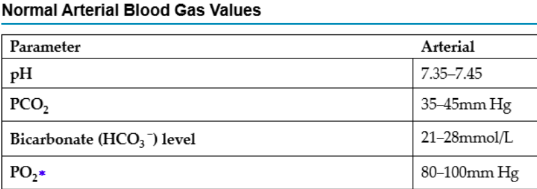
What does PaCO2 measure to?
Measures the amount of carbon dioxide in the arterial blood. Indicates how well the lungs are excreting/retraining CO2 (carbonic acid)
a lot = acidotic, too little =alkalotic
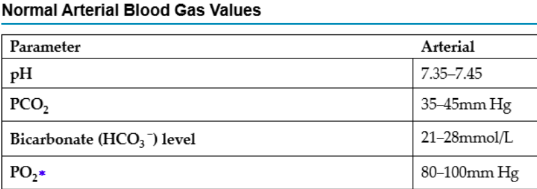
What does HCO3 refer to?
Measures the amount of bicarbonate in the arterial blood
Indicates how well the kidneys are excreting/retaining bicarbonate (a lot of bicarbonate = alkalotic, little = acidotic)
What does PaO2 measure?
Measures the amount of oxygen in the arterial blood
→ low = hypoxemia
How do you evaluate an ABG?
pH
Is it acidic, alkalotic, or normal?
PaCO2
If greater than 45mmHg, this tells us there is a lot of carbonic acids – acidic
If it is lower than 35mmHg, this tells us there is not enough acids – alkalotic
If it is in the normal range - normal
HCO3
If greater than 28mmol/L, this tells us there are a lot of bicarbonates – alkalotic
If it is lower than 21mmol/L, this tells us there are not
enough bicarbonates – acidotic
If it is in the normal range - normal
Does PCO2 or HCO3 match the pH?
Whichever substance matches the pH, it helps us determine the cause (respiratory or metabolic)
Compensation
Determine if body is attempting to compensate
a. If compensatory mechanisms are
functioning, the pH will try to move towards 7.35-7.45
When there is true compensation, pH will
be normal but other values are abnormal
When pH is abnormal and PCO2 or HCO3 are going in opposite directions (one acidotic, one alkalotic) – there is compensation occurring however not full compensation – this is partial compensation
Oxygenation
Assess the PaO2 and O2
If abnormal, hypoxemia is present