Parasites and Drugs
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
What is a definitive host?
The host in which the parasite reaches sexual maturity. Examples: mosquito is definitive host for malaria; human is definitive host for Schistosoma.
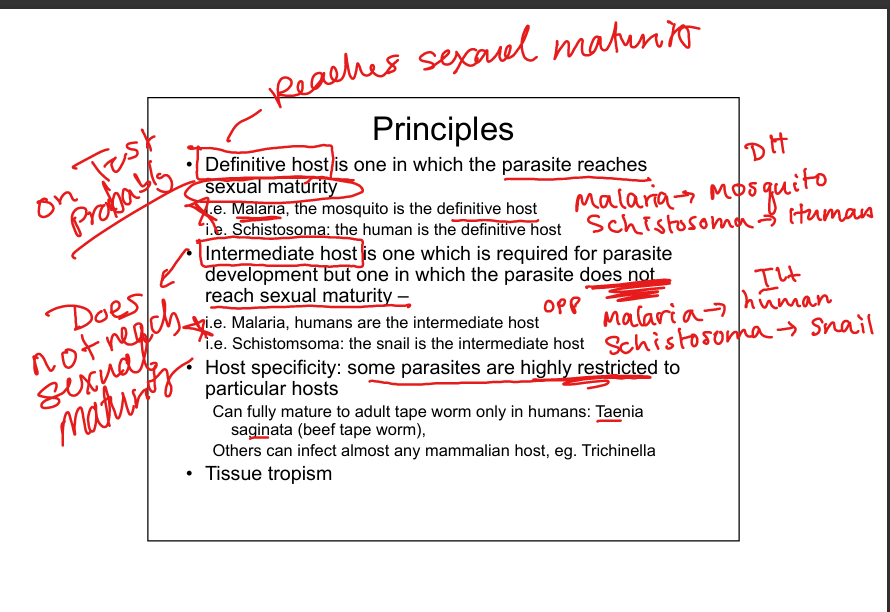

What is an intermediate host?
The host required for parasite development but where sexual maturity does not occur. Examples: humans are intermediate hosts for malaria; snails are intermediate hosts for Schistosoma.


What are intestinal protozoa of importance?
Entamoeba histolytica, Giardia lamblia, Cryptosporidium(saved for GI block). All are single‑celled eukaryotes with distinct life cycles and transmission routes.
Describe Entamoeba histolytica transmission and life cycle.
Fecal‑oral via contaminated food/water. Ingested cysts (environmentally resistant, 4 nuclei) → trophozoites in colon. Trophozoites invade mucosa → colitis, ulcers, dysentery. Can spread to liver (abscess) or brain. Trophozoites encyst → cysts passed in stool. Life cycle: cyst → trophozoite → cyst.


What are Entamoeba histolytica virulence factors?
Galactosamine adherence lectin (attachment), proteinases (tissue destruction), lysis of WBCs. Pathogenesis requires penetration of mucosal barrier. Host defects (malnutrition, impaired mucosa) predispose to invasive disease.


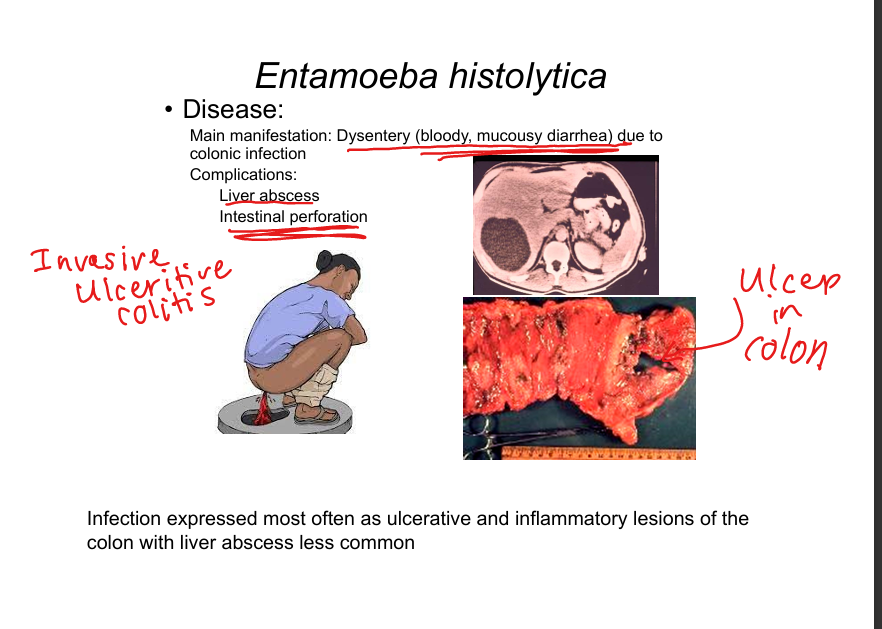
What disease does Entamoeba histolytica cause?
Ulcerative colitis with bloody diarrhea (dysentery). Complications: liver abscess, intestinal perforation. Diagnosis: stool antigen tests, microscopy (trophozoites ingesting RBCs).

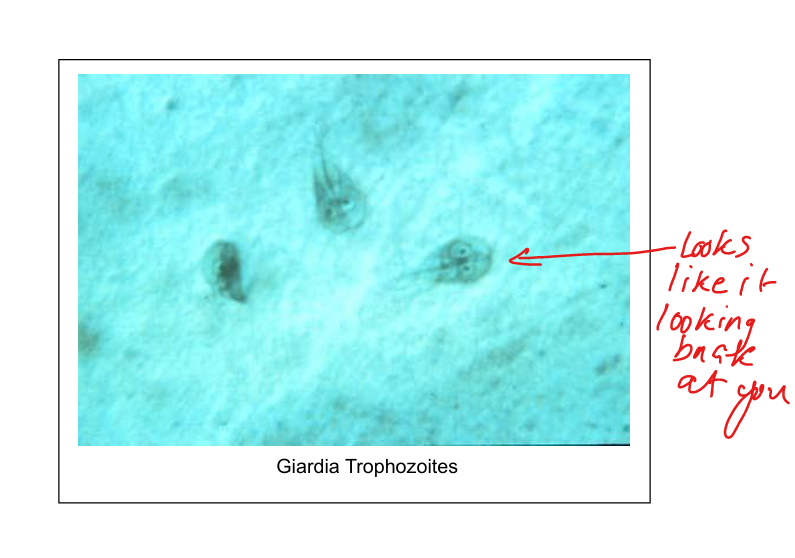
Describe Giardia lamblia transmission and life cycle.
Worldwide distribution; reservoirs include mammals (beaver). Transmission: contaminated water, food, person‑to‑person, zoonosis. Ingested cysts → trophozoites in small intestine(duodenum/jejunum) attach to villi → flatten microvilli → malabsorptive diarrhea. Trophozoites encyst → cysts passed in stool. Life cycle: cyst → trophozoite → cyst. No Blood No Fever


What are Giardia lamblia virulence and host defenses?
Attachment to villi causes microvilli destruction. Does not invade mucosa. Host defense = IgA. IgA deficiency predisposes to chronic giardiasis.


What disease does Giardia lamblia cause?
Watery, malabsorptive diarrhea (acute, subacute, chronic). No blood, no fever. Diagnosis: stool microscopy (cysts/trophozoites with “face‑like” appearance).

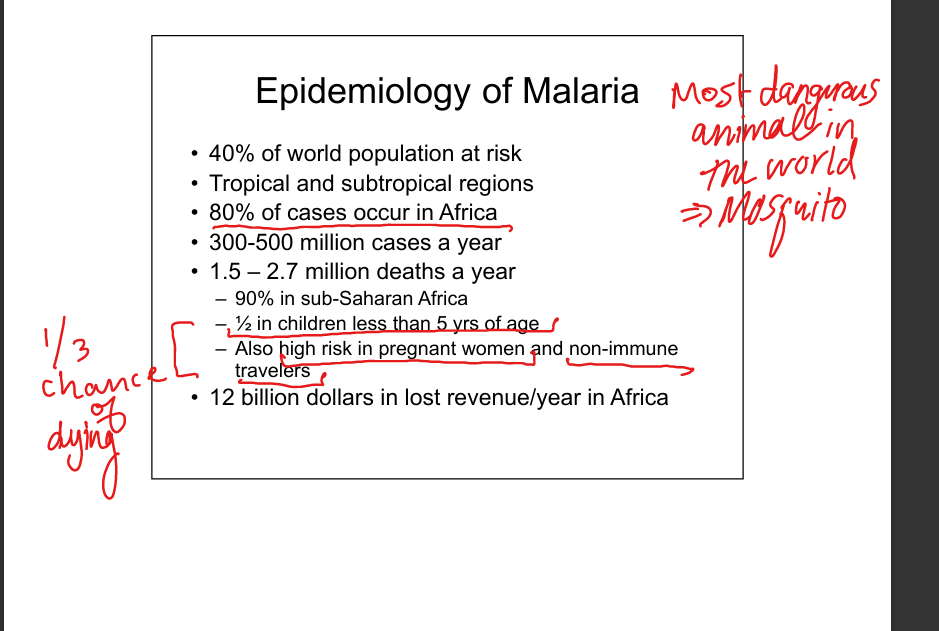
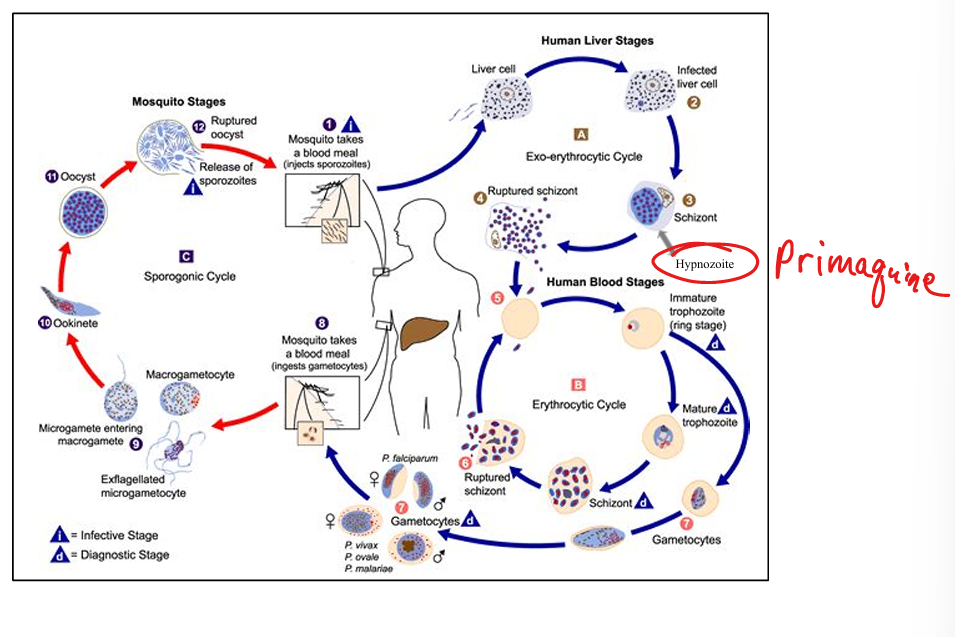
Describe malaria epidemiology.
Highly prevalent in Africa, children <5, pregnant women, non‑immune travelers: 1/3 chance of dying. Caused by Plasmodium species.

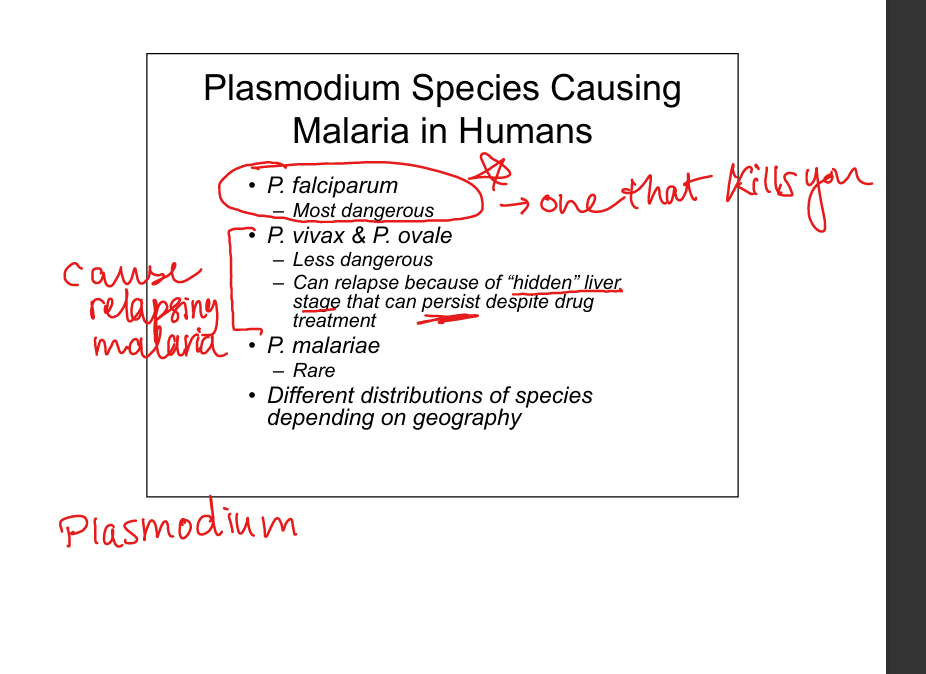
Which Plasmodium species infect humans?
P. falciparum (most lethal, cerebral malaria, high mortality). P. vivax & P. ovale (relapsing malaria due to dormant liver hypnozoites). P. malariae (rare).

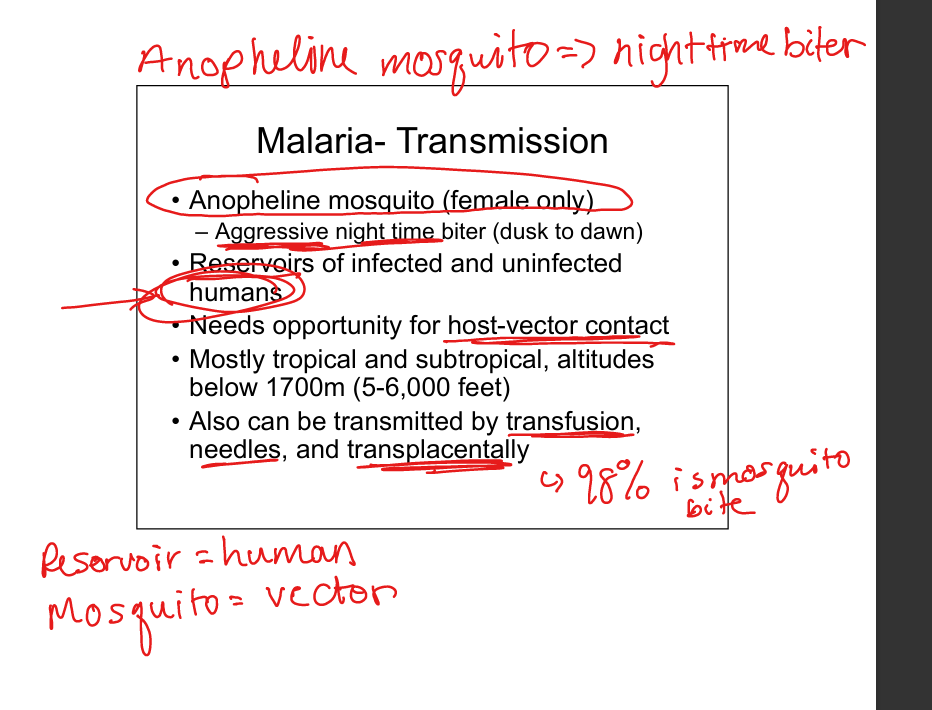
Describe malaria transmission.
Female Anopheline mosquito (night‑time biter). Reservoir = humans. Also via transfusion, needles, transplacental. Vector‑host contact required.

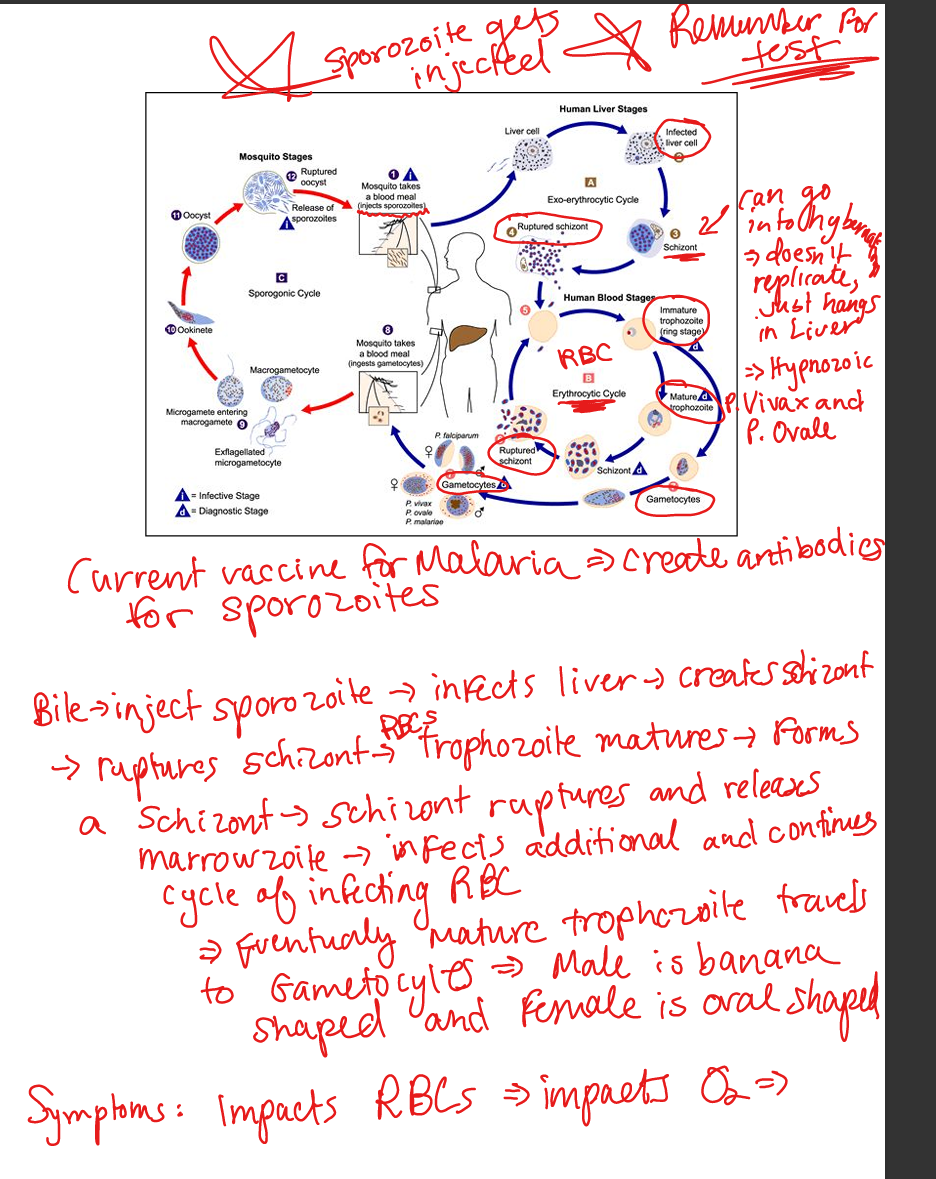
Outline malaria life cycle.
(1) Mosquito injects sporozoites → liver (exoerythrocytic schizogony). (2) Schizonts rupture → merozoites infect RBCs (erythrocytic schizogony). (3) RBC trophozoites mature → schizonts → rupture → more merozoites. (4) Some form gametocytes → ingested by mosquito. (5) In mosquito: gametocytes → zygote → ookinete → oocyst → sporozoites → salivary glands.

What is the pathogenesis of falciparum malaria?
P. falciparum causes microvascular occlusion via cytoadherence (“knobs” on RBCs). »Tissue hypoxia»Leads to cerebral malaria, renal failure, hypoglycemia, systemic inflammation. High parasite load = high mortality.


How is malaria diagnosed?
Blood smear (Giemsa/Wright stain) showing ring forms, trophozoites, gametocytes. Antigen detection (rapid tests). PCR. Clinical suspicion in febrile patient with travel to endemic area.


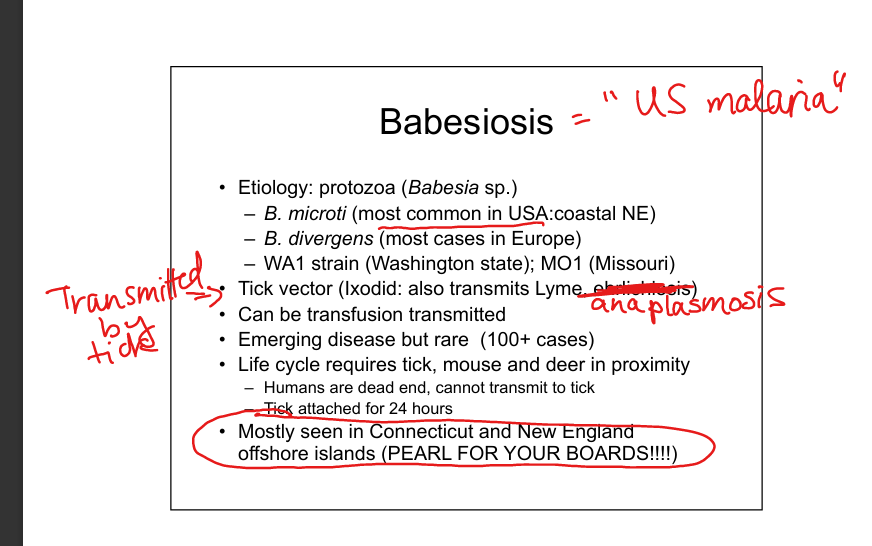
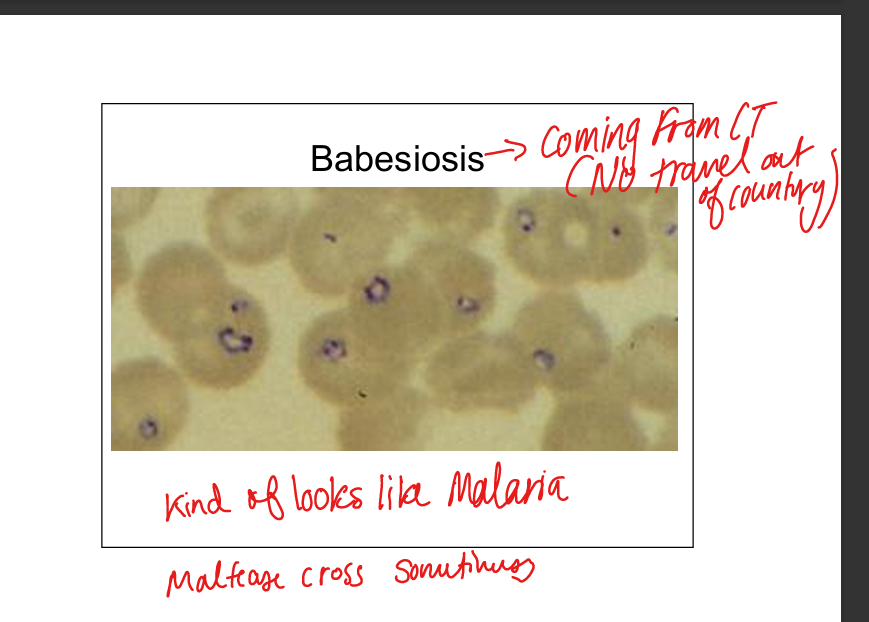
Describe Babesia infection.
Protozoa (B. microti in USA, B. divergens in Europe). Tick vector (Ixodes, also transmits Lyme, Anaplasma). Humans are dead‑end hosts. Transmission via tick bite or transfusion. Endemic in New England offshore islands. Disease resembles malaria (fever, hemolysis, “Maltese cross” on smear). SPLENECTOMY LIST

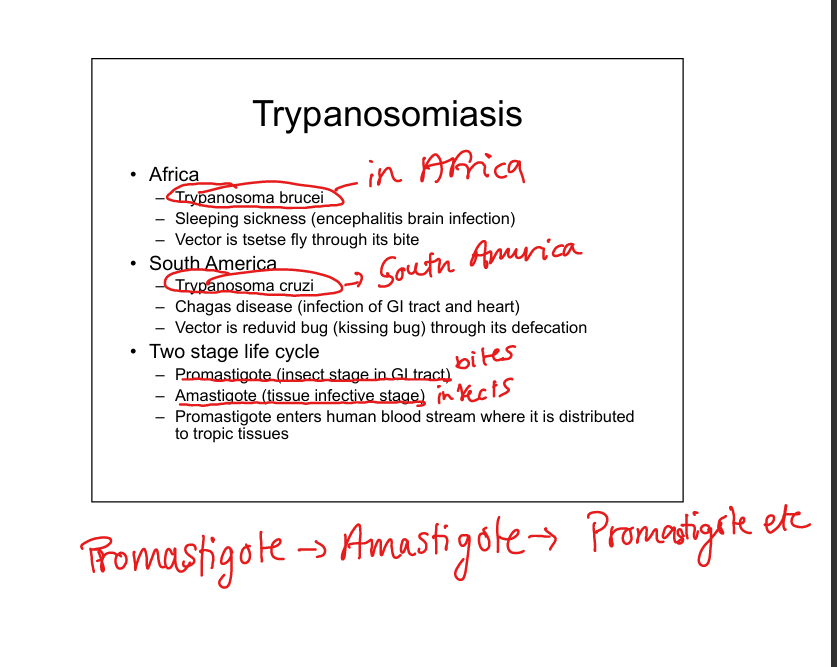
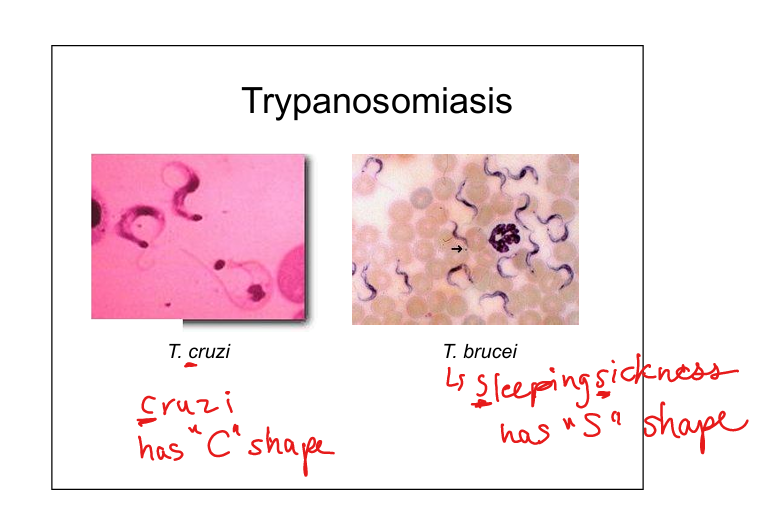
Describe Trypanosoma infections (T. brucei and T cruzi)
T. brucei (African sleeping sickness, encephalitis; vector = tsetse fly). T. cruzi (Chagas disease, GI and cardiac involvement; vector = reduviid “kissing bug” via fecal contamination). Life cycle: insect stage (promastigote) → human tissue stage (amastigote)—>Promastigote PRO GOES FIRST


Describe Toxoplasma gondii infection.
Definitive host = cats. Transmission: ingestion of undercooked meat or cat feces. Usually asymptomatic. In immunocompromised (AIDS): encephalitis, myocarditis, myositis. Congenital infection (esp. 1st trimester): chorioretinitis, cerebral calcifications, disseminated disease. Prevention: cook meat, avoid cat litter in pregnancy.


Describe hookworm infection.
Ancylostoma duodenale (Mediterranean, Asia), Necator americanus (Americas, Africa, SE Asia). Transmission: skin contact with contaminated soil. Life cycle: larvae penetrate skin → venous system → lungs → trachea → swallowed → mature in small intestine → attach to mucosa → chronic blood loss → iron deficiency anemia. Eggs in stool hatch in soil. Clinical: Iron deficiency anemia, eosinophilia.


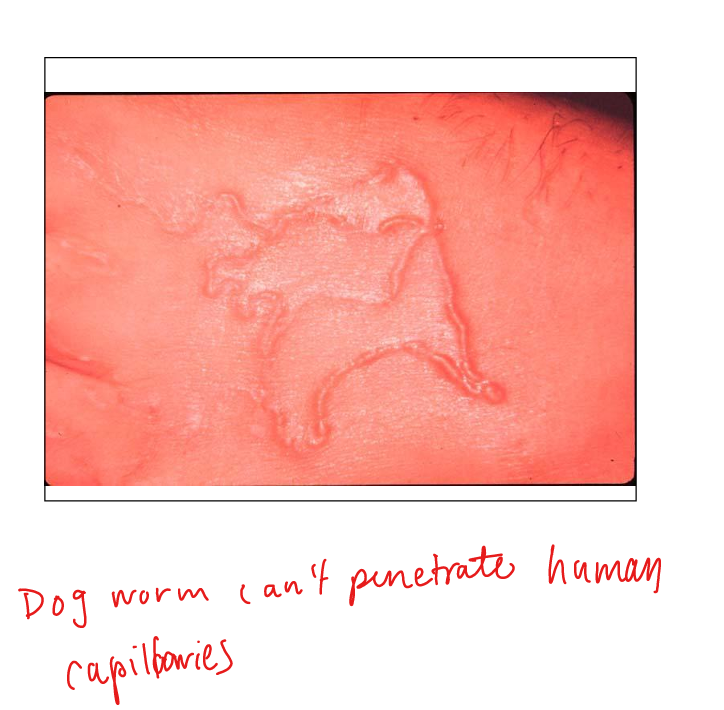
What is cutaneous larva migrans?
Accidental human infection by dog/cat hookworm larvae. Larvae penetrate skin, migrate in cutaneous tract → pruritic serpiginous lesions. Seen in tropics, SE USA. Prevention: wear shoes.


Describe schistosomiasis (Blood Flukes)
Blood flukes. S. mansoni (GI venous plexus → periportal fibrosis, portal hypertension, cirrhosis). S. haematobium (bladder venous plexus → hematuria, bladder wall fibrosis, squamous cell carcinoma). Transmission: cercariae from snails penetrate skin in contaminated water. Pathogenesis: granulomatous inflammation around eggs → fibrosis. Eosinophilia common. Cirrhosis of the liver in 20-30 yrs and rock hard bladder


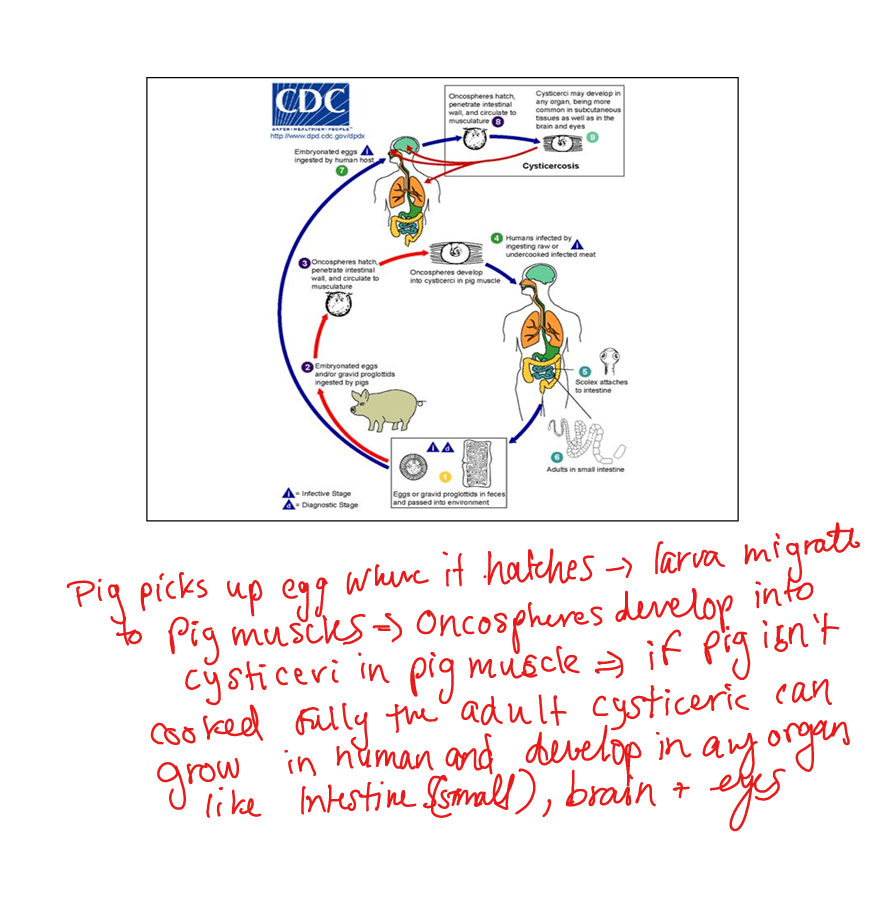
Describe Taenia infections.
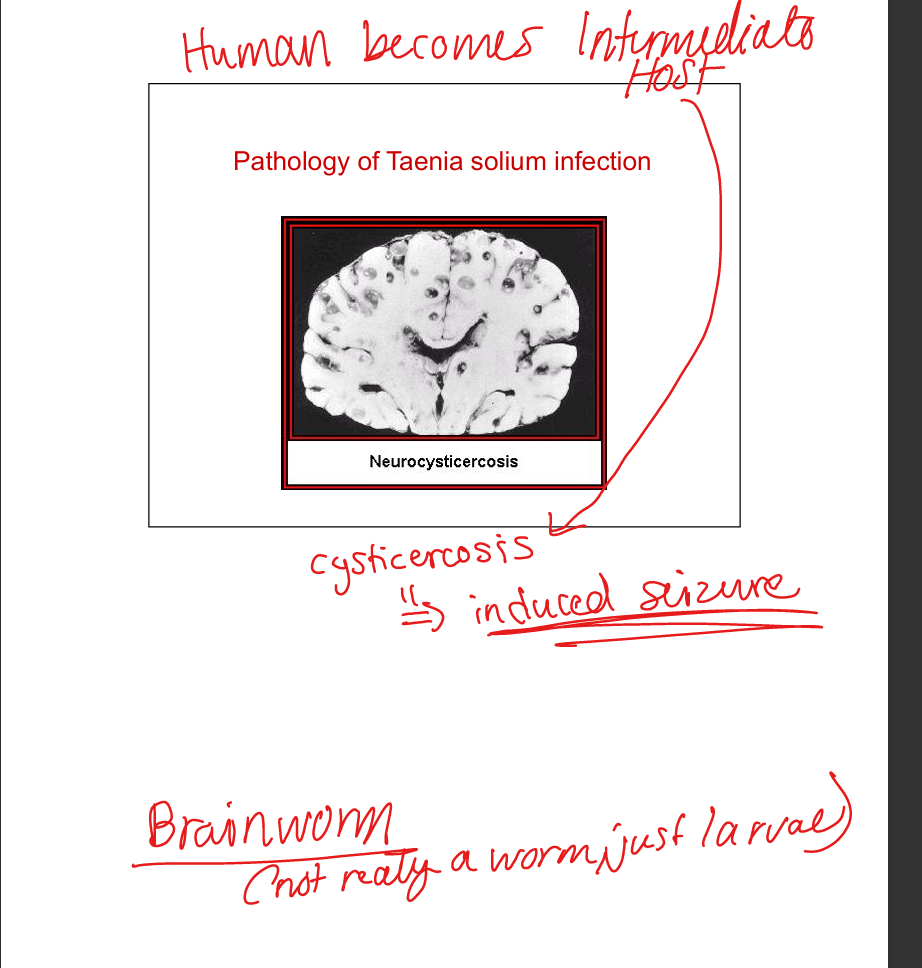
T. saginata (beef tapeworm). T. solium (pork tapeworm). Transmission: ingestion of undercooked meat with cysticerci. Adult tapeworms cause mild disease. Ingestion of T. solium eggs → cysticercosis (larvae in brain, eyes, muscle). Neurocysticercosis → seizures. Diagnosis: imaging, serology. Prevention: cook pork thoroughly.

Chloroquine
Mechanism: 4‑aminoquinolone; inhibits heme polymerase → toxic heme buildup kills parasite. Resistance: drug efflux from food vacuole. Active stage: erythrocytic only (not liver, not hypnozoite). Indications: P. falciparum (non‑resistant areas: Haiti, Central America, Middle East), P. vivax, P. ovale, P. malariae. Toxicity: generally well tolerated. Oral, long half‑life, weekly prophylaxis.


Mefloquine (Lariam®)
Mechanism: not fully understood; blood schizonticide. Resistance: rare, increasing in SE Asia (Cambodia/Thailand). Active stage: erythrocytic only. Indications: chloroquine‑resistant P. falciparum, prophylaxis. Toxicity: nausea/vomiting, vivid dreams/nightmares, CNS stimulation (anxiety, tremor, psychosis, seizures). Contraindicated in seizure/psychiatric disorders. Oral, weekly dosing.


Atovaquone/Proguanil (Malarone®)
Mechanism: combined drug; active against erythrocytic and liver schizonts. Resistance: not yet major issue. Active stage: erythrocytic + liver schizonts (not hypnozoites). Indications: non‑severe falciparum malaria, causal prophylaxis, resistant strains. Toxicity: well tolerated, oral, daily dosing. Most useful prophylactic agent. Malarone=two for one special:gets both Liver Schizont and Erythrocytic


Doxycycline
Mechanism: antibacterial; inhibits protein synthesis. Active stage: erythrocytic only. Indications: resistant malaria prophylaxis, combined with quinine/quinidine for treatment. Toxicity: photosensitivity (sunburn risk). Oral, daily dosing.


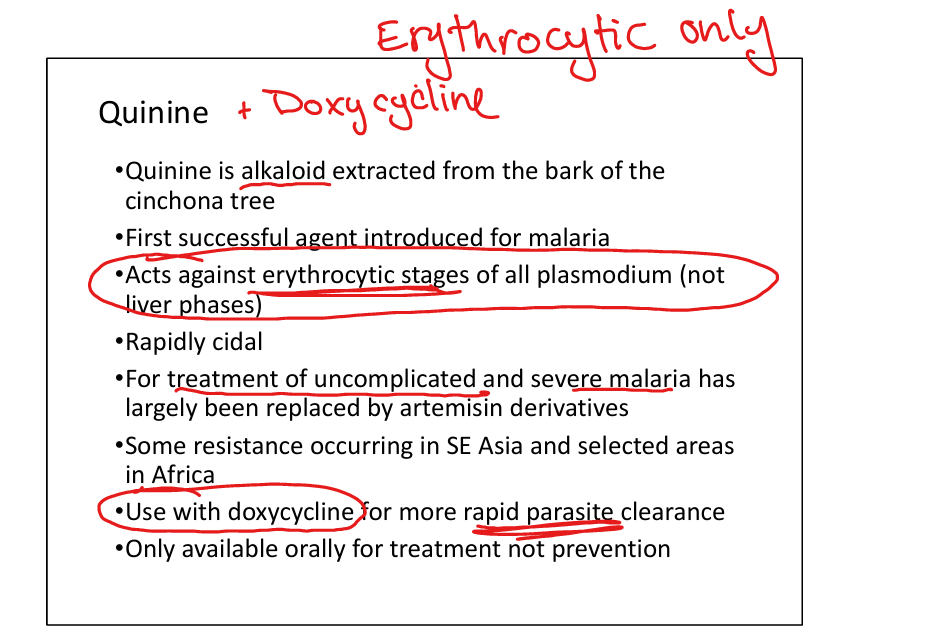
Quinine/Quinidine
Mechanism: alkaloid from cinchona bark; rapidly cidal against erythrocytic stages. Resistance: some in SE Asia/Africa. Active stage: erythrocytic only. Indications: treatment of uncomplicated/severe malaria (replaced largely by artemisinins). Toxicity: cinchonism (tinnitus, headache, nausea), hypoglycemia. Oral only, used with doxycycline for faster clearance.

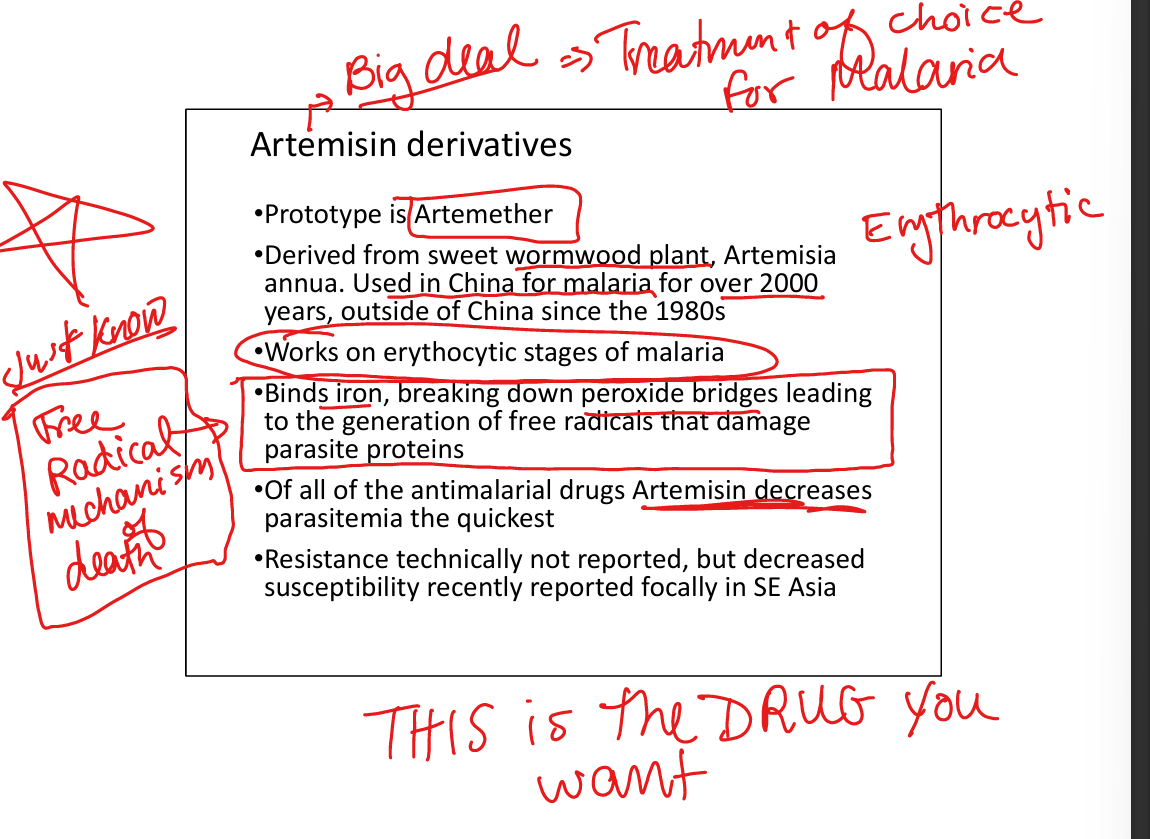
Artemisinin derivatives (e.g. Artemether)
Mechanism: binds iron, generates free radicals → parasite protein damage. Resistance: decreased susceptibility reported in SE Asia. Active stage: erythrocytic only. Indications: treatment of falciparum malaria (fastest parasite clearance). Toxicity: generally well tolerated. WHO mandates combination therapy (ACT: artemisinin + lumefantrine/pyrimethamine) to prevent resistance. Oral ACT FDA‑approved in US; IV available for severe malaria. (Art is for free radicals)

Primaquine *****(hypnotizing)*****
Mechanism: active against liver stages including hypnozoites. Resistance: none noted. Active stage: hepatic schizonts + hypnozoites. Indications: terminal prophylaxis for P. vivax and P. ovale (prevent relapse), causal prophylaxis. Toxicity: hemolysis in G6PD deficiency → must check G6PD before prescribing. Oral, daily dosing.


Metronidazole
Mechanism: nitroimidazole; forms free radicals that damage DNA. Indications: amoeba (Entamoeba histolytica), Giardia lamblia. Toxicity: GI upset, metallic taste, disulfiram‑like reaction with alcohol. Used with paromomycin for cyst eradication in amoeba.


Drug(s) against worms
Names: Mebendazole, Albendazole, Praziquantel, Ivermectin. Indications: “worms” (nematodes, cestodes, trematodes). Mechanisms: vary (microtubule inhibition, tegument disruption, chloride channel activation). Toxicities: generally well tolerated; GI upset most common.

