Development & Isotopes of Atoms
1/25
Earn XP
Description and Tags
Development of the Atom Dalton, Thomson, Rutherford Subatomic Particles Isotopes Average Atomic Mass
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
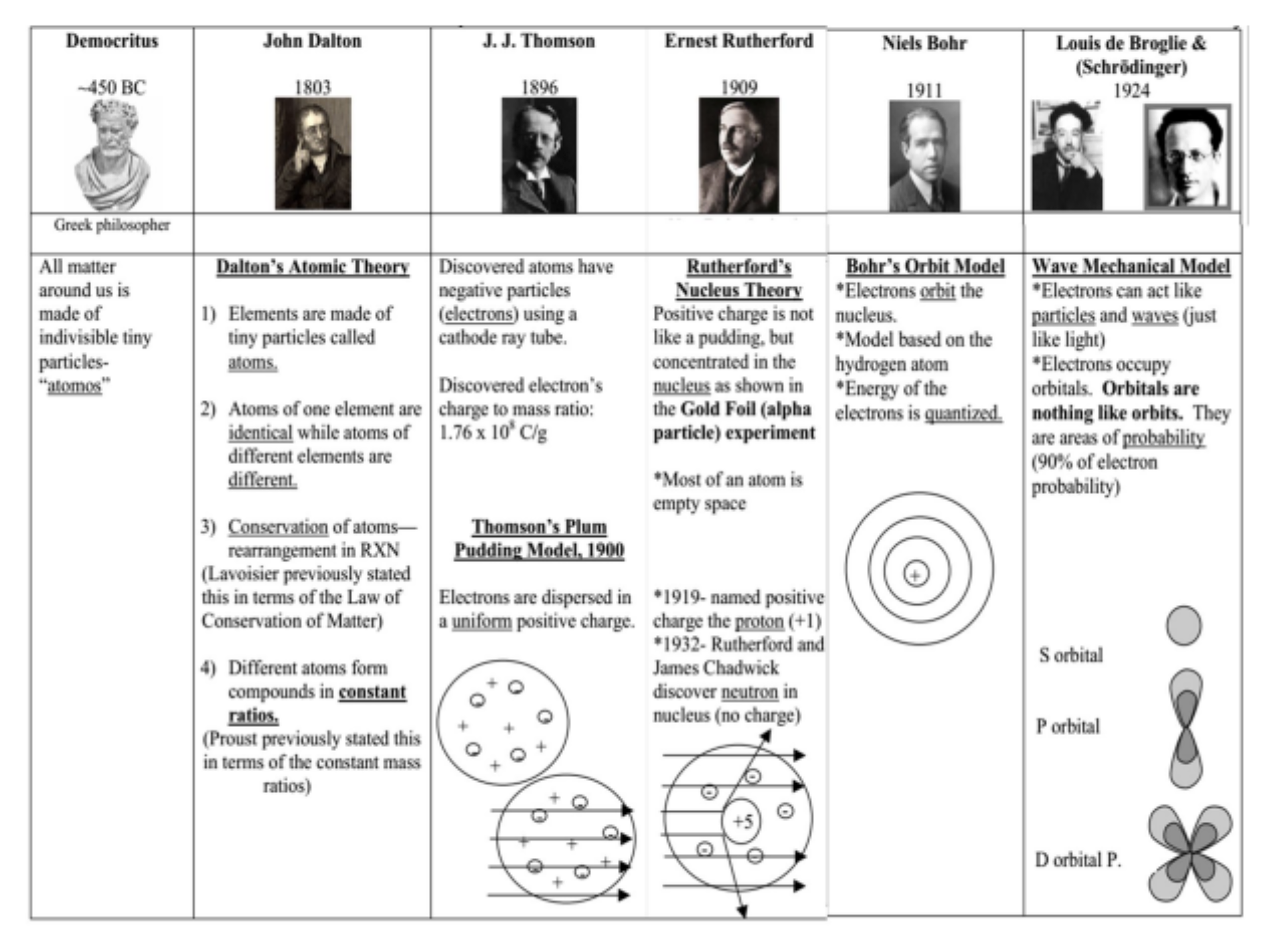
democritus (~450 bc)
greek philosopher
all matter around us is made of indivisible tiny particles-
"atomos”
john dalton (1803)
described atoms as indivisible, indestructible, solid, and uniform spheres
“billiard ball model”
john dalton’s atomic theory five postulates
1. Each element is composed of tiny, indestructible particles called atoms.
2. All atoms of a given element have the same mass and chemical properties.
3. Atoms of different elements are different.
4. Atoms combine in simple, whole-number ratios to form compounds.
5. Atoms of one element cannot change into atoms of another element. In a chemical reaction, atoms only change the way that they are bound together with other atoms.
john dalton’s theory summarized
elements are made of tiny particles called atoms.
atoms of one element are identical while atoms of different elements are different.
conservation of atom rearrangement in chemical reactions (lavoisier previously stated this in terms of the law of conservation of matter)
different atoms form compounds in constant ratios. (proust previously stated this in terms of the constant mass ratios)
j.j thomson (1896)
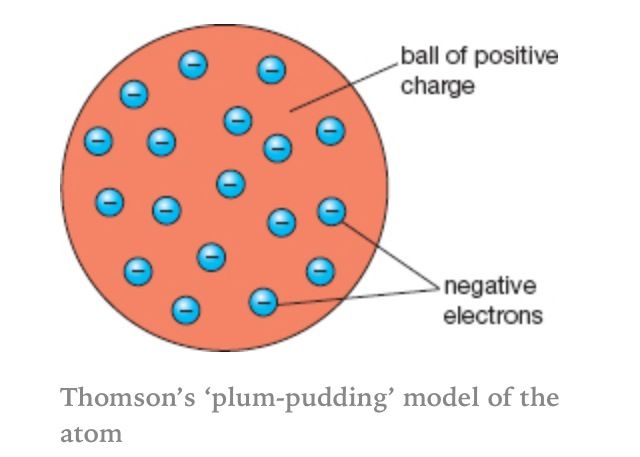
electrons are dispersed in a uniform positive charge
“plum pudding model” 1990
depicts the atom as a diffuse, positively charged sphere with negatively charged electrons embedded within it
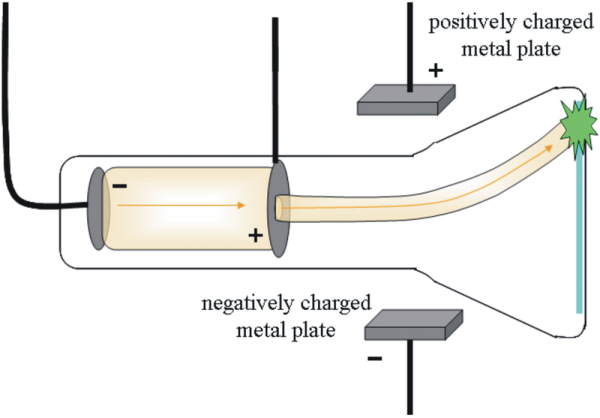
j.j thompson’s experiment
to test the properties of the particles, thomson placed two oppositely-charged electric plates around the cathode ray. the cathode ray was deflected away from the negatively-charged electric plate and towards the positively-charged plate. this indicated that the cathode ray was composed of negatively-charged particles.
attracted to positive end = negative
thomson also placed two magnets on either side of the tube, and observed that this magnetic field also deflected the cathode ray. the results of these experiments helped thomson determine the mass-to-charge ratio of the cathode ray particles, which led to a fascinating discovery the mass of each particle was much, much smaller than that of any known atom. thomson repeated his experiments using different metals as electrode materials, and found that the properties of the cathode ray remained constant no matter what cathode material they originated from. from this evidence, thomson made the following conclusions:
the cathode ray is composed of negatively-charged particles.
the particles must exist as part of the atom, since the mass of each particle is only the mass of a hydrogen atom.
these subatomic particles can be found within atoms of all elements.
j.j thompson’s discoveries
discovered atoms have negative particles (electrons) using a cathode ray tube
discovered electron's charge to mass ratio: 1.76 x 10 C/g
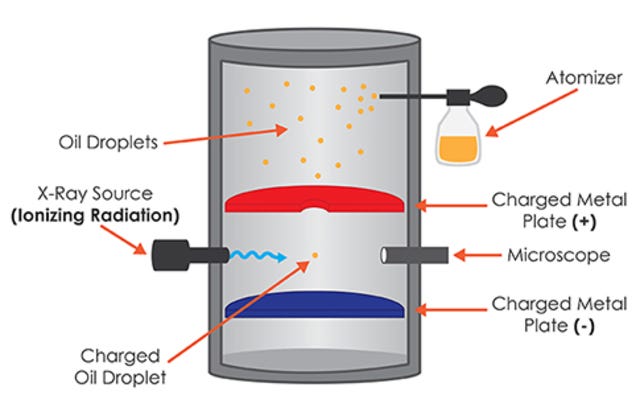
millikan’s oil drop experiment (1909)
measured the fundamental charge of an electron by suspending charged oil droplets in an electric field and observing their motion
charge of an e- is 1.60 x 10-19C
ernest rutherford (1909)
an atom has a positive, heavy, tiny nucleus with electrons moving around it.
most of an atom is empty space
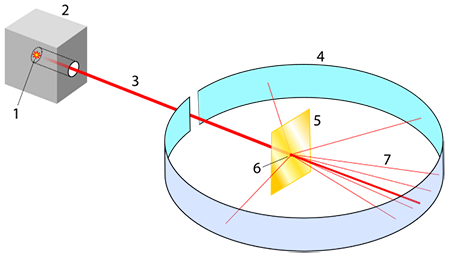
the gold foil experiment
rutherford shot alpha particles at a thin sheet of foil
conclusions:
most of the alpha particles passed straight through the foil = the atom is mostly open space
some of the alpha particles were deflected at large angles, and some were reflected = the atom contains a small, heavy, positive central nucleus.
james chadwick (1932)
discovered the neutron
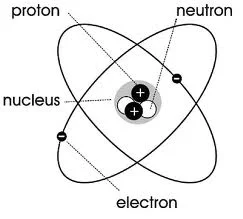
proton
mass (kg) = 1.67262 × 10^-27
mass (amu) = 1.00727
charge (relative) = +1
charge (C) = +1.60218 × 10^-19
neutron
mass (kg) = 1.67493 × 10^-27
mass (amu) = 1.00866
charge (relative) = 0
charge (C) = 0
electron
mass (kg) = 0.00091 × 10^-27
mass (amu) = 0.00055
charge (relative) = -1
charge (C) = -1.60218 × 10^-19
all developments of atoms timelines + major discoveries
atomic mass number
the number of protons in an element
unique to each element
ions
an atom or a group of atoms that has a net electric charge due to a loss or gain of electrons
non-zero charge
charge is written in the upper right hand corner
e.g) Na⁺, S²⁻, CO₃²⁻
isotopes
atoms of the same element with different masses
isotopes have different numbers of neutrons
a sample of an element in nature consists of a mixture of its naturally occurring isotopes
isotopic notation
e.g) ¹⁴₆C
can use to find number of neutrons (mass number - number of protons = number of neutrons)
can also be written as: element name - mass number
e.g) carbon-14
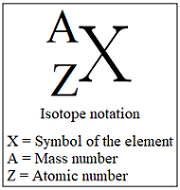
mass number vs atomic mass
mass number: the total number of protons and neutrons in the nucleus of a single isotope of an element
whole number
atomic mass: the weighted average of the atomic masses of all the naturally occurring isotopes of an element
decimal, on periodic table
atomic mass
is a weighted average of all isotopes, resulting in fractional values
mass and percentage (natural abundance) of each isotope is determined from mass spectrometry
mass spectrometry
technique used to identify and quantify compounds by measuring the mass-to-charge ratio (m/z) of ionized molecules
atoms are converted to positively charged ions, accelerated and passed through magnetic field that deflects their path
heaviest ions (most neutrons) undergo the least deflection
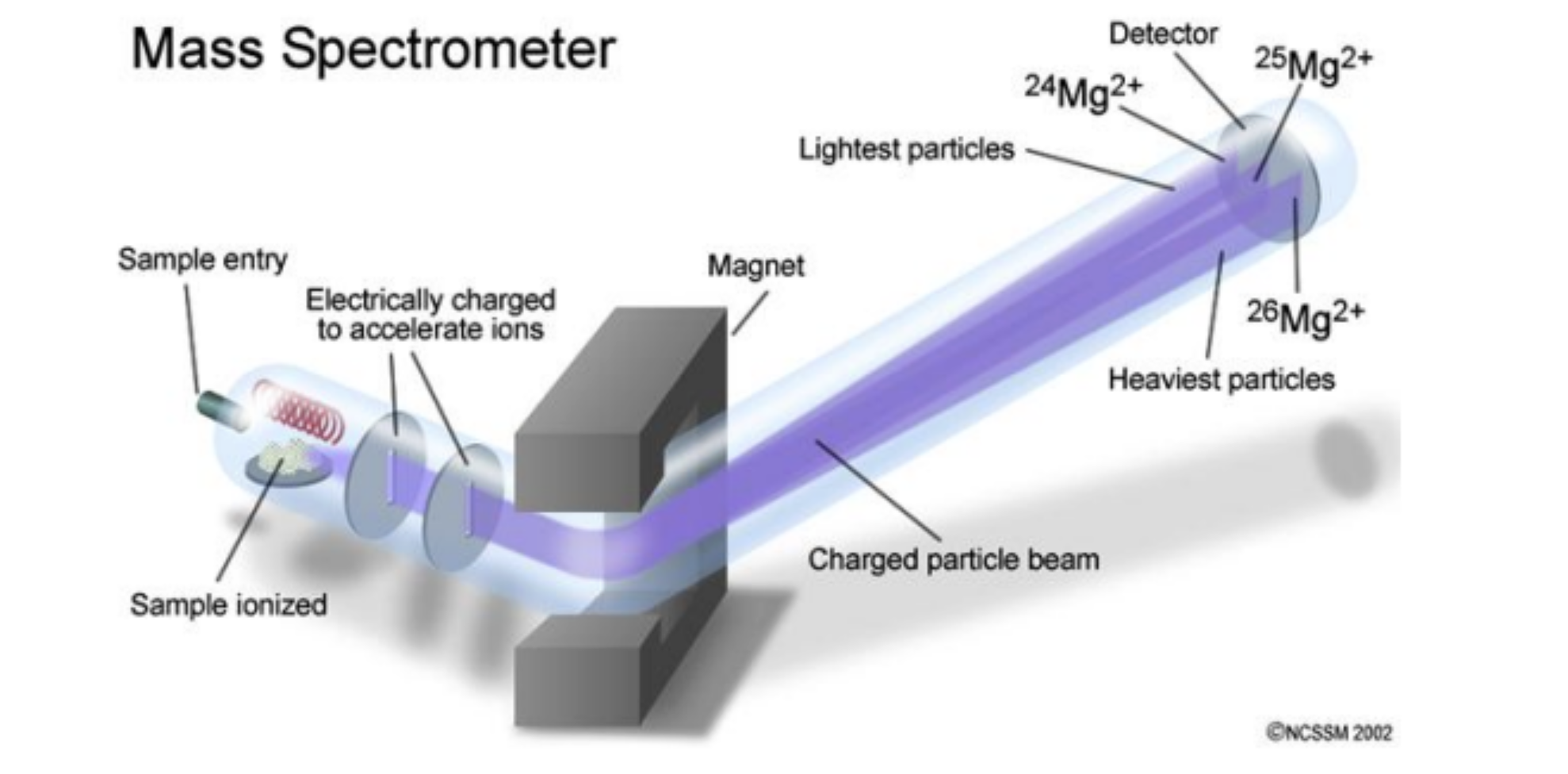
mass spectrometry graph
(y-axis) relative/natural abundance = the height of each bar on the y-axis, showing how common each ion is relative to the most abundant ion
(x-axis) m/z = the value on the x-axis, representing the mass of an ion divided by its charge
isotopes have different m/z ratio
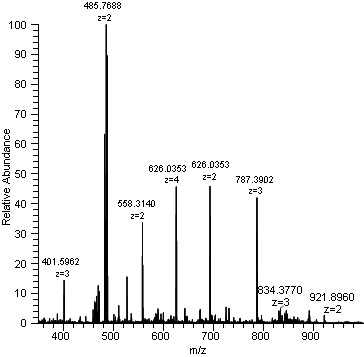
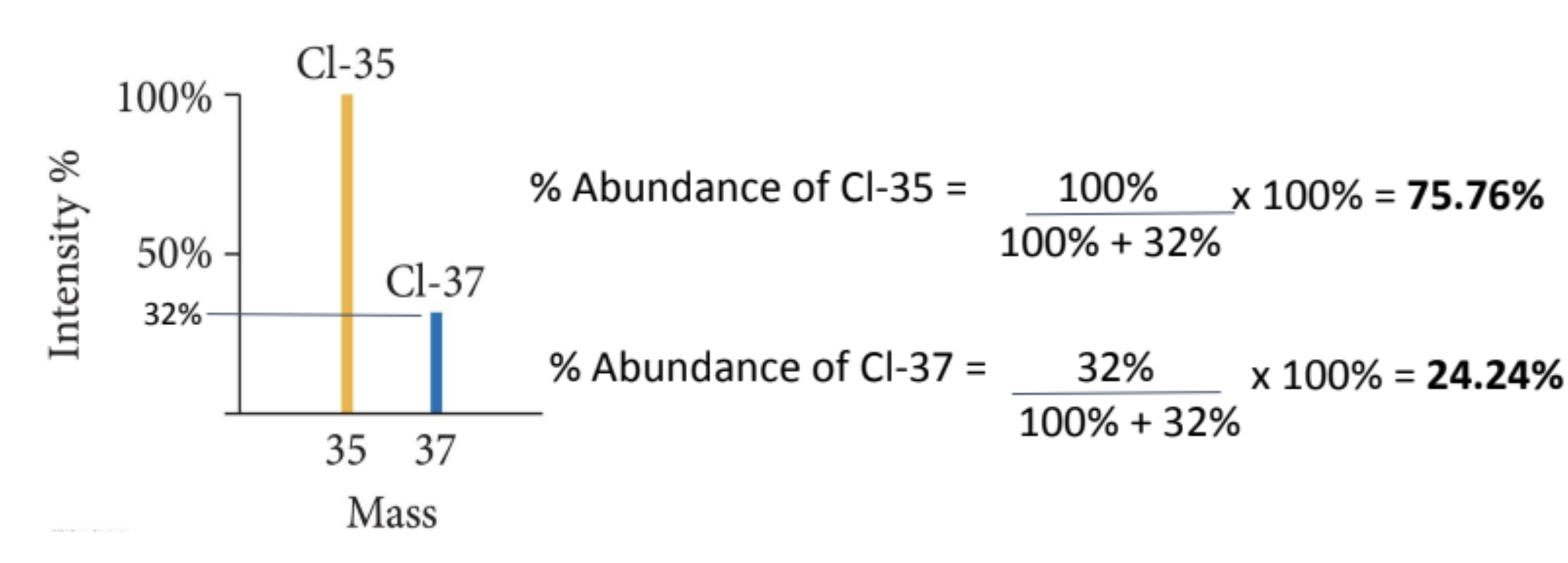
intensity
most abundant isotope set to 100 (base peak)
other isotopes expressed as a percentage relative to this base peak and relative abundance compared to the most abundant ion
intensity to abundance equation
% abundance of a isotope = (intensity of the peak / total intensity of all peaks) 100
e.g)
% abundance of Cl-35 = 100% /(100% + 32%) x 100 = 75.76%
% abundance of Cl-37 = 32% / (100% + 32%) x 100 = 24.24%
atomic mass formula
atomic mass = (mass of isotope a)(%a) + (mass of isotope b)(b%) + …
remember to convert percentage into decimal by dividing by 100
no significant figures needed