ionic bonding topic 12
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
In A⁺X⁻ type ionic solids, what must be true about the coordination numbers
The CNs of A⁺ and X⁻ must be identical.
the geometry does NOt need to be the same
what are the two types of holes in close-packed structures?
tetrahedral holes
octahedral holes
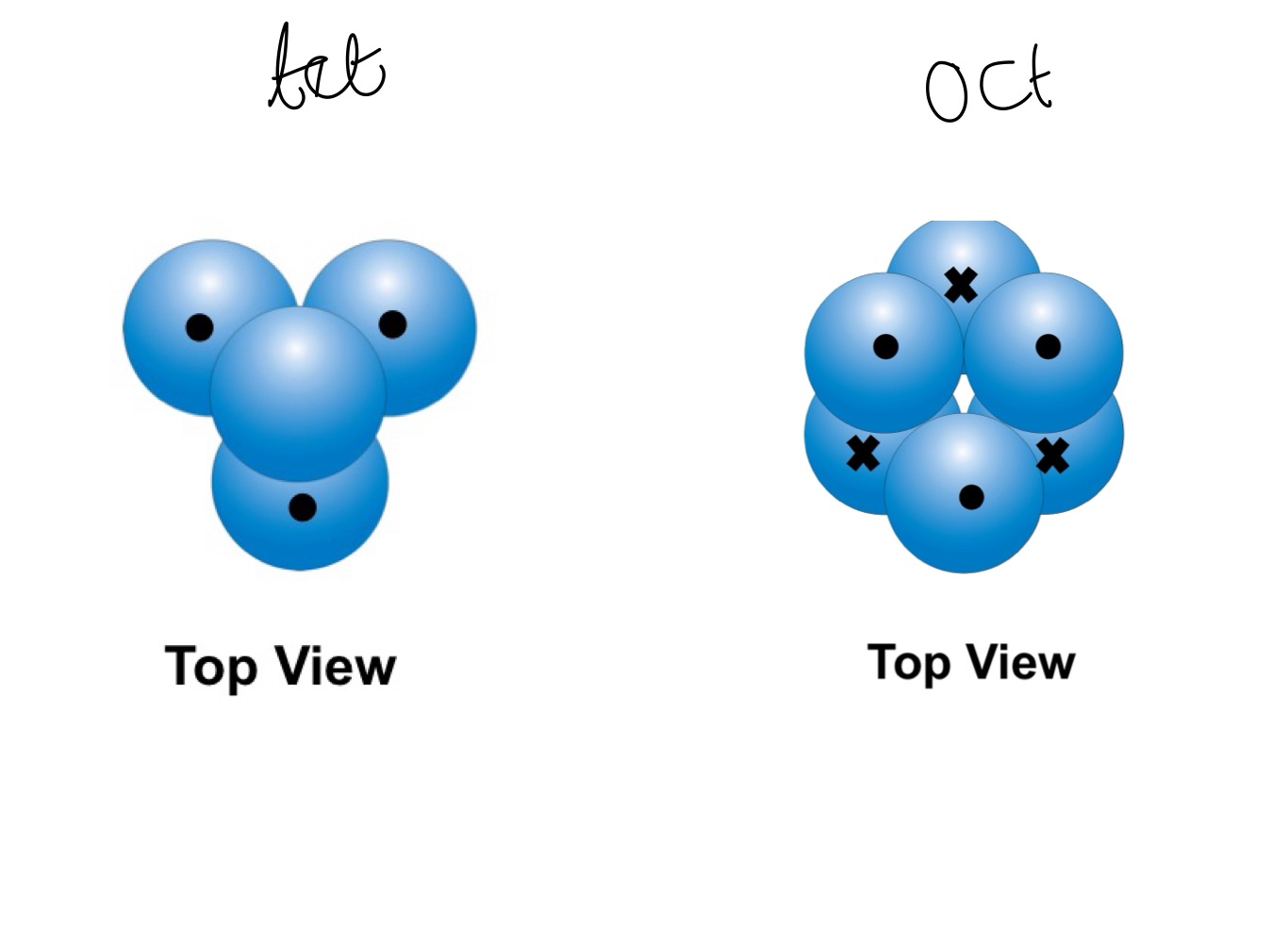
when does a tetrahedral hole occur ?
where a sphere from one layer is over the centre of a triangle in the layer above or below
when does an octahedral hole occur ?

top view of an tetrahedral hole vs an octahedral one
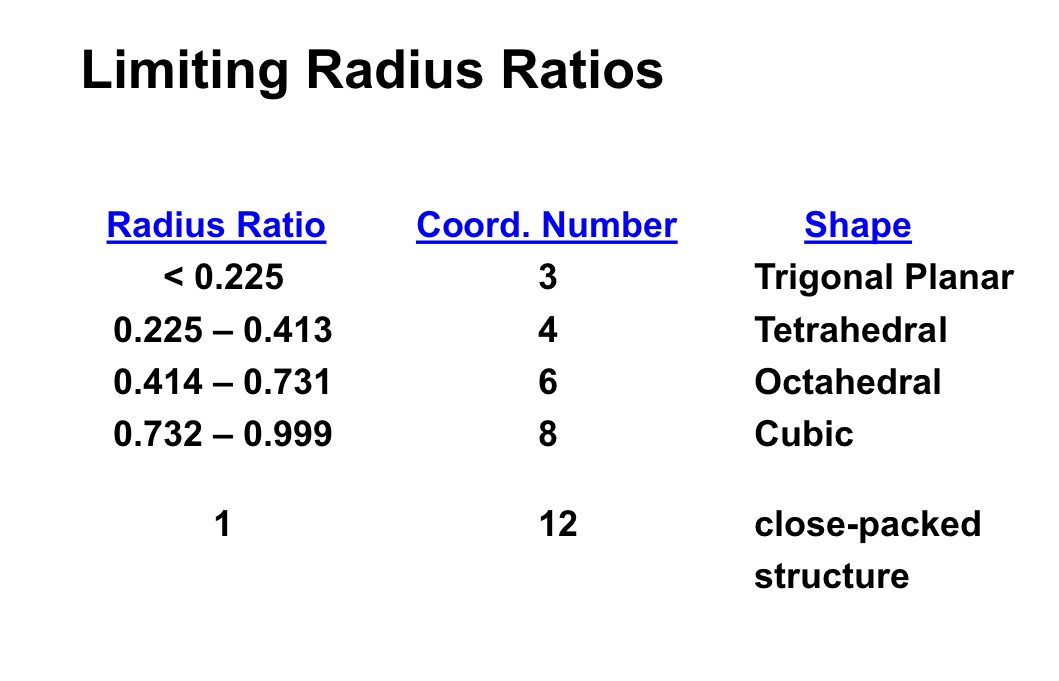
for Ax structures what ratio do you need?
1:1
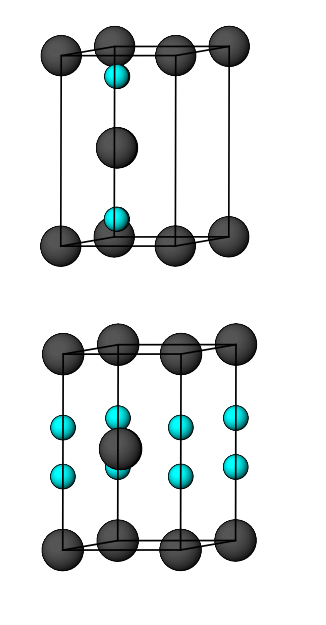
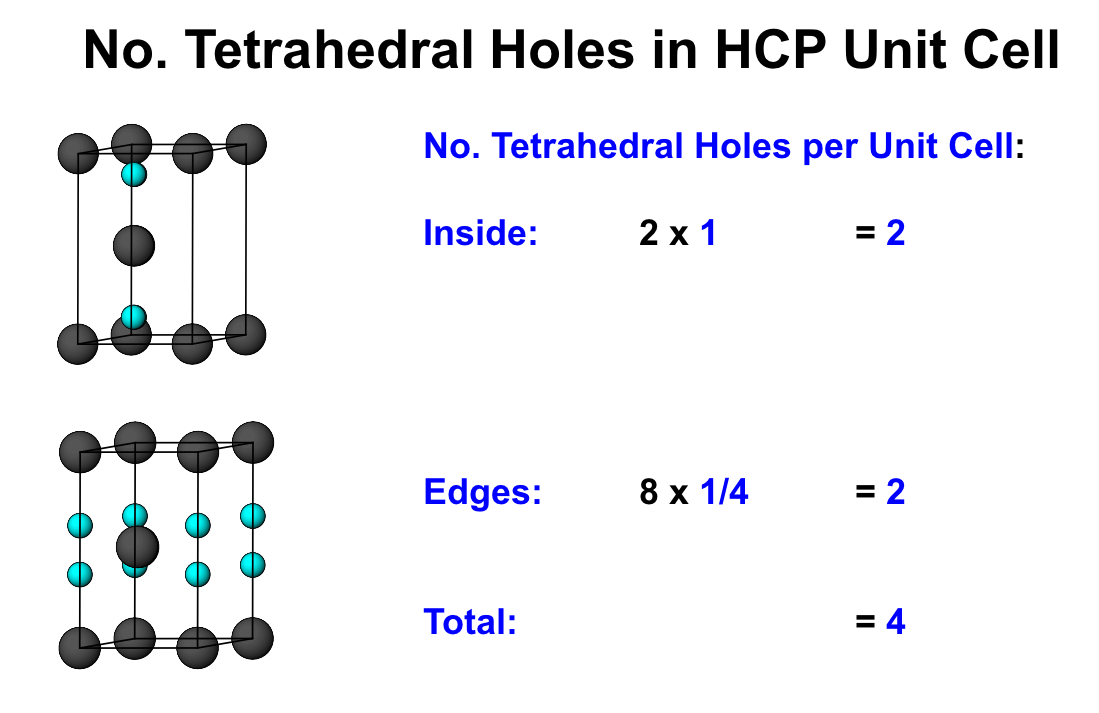
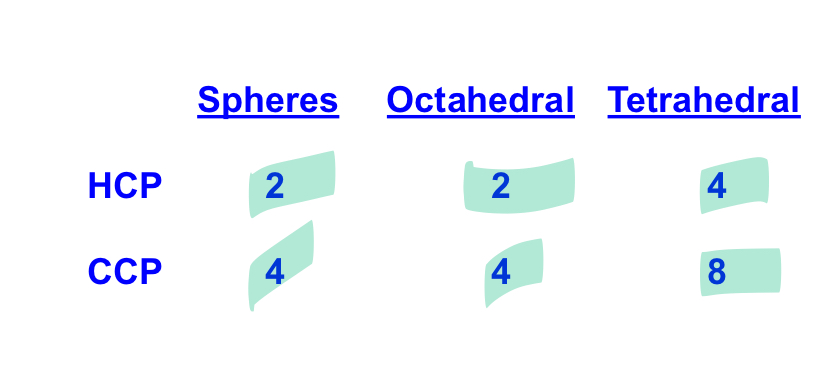
what is the number of tetrahedral holes per unit cell for HCP?
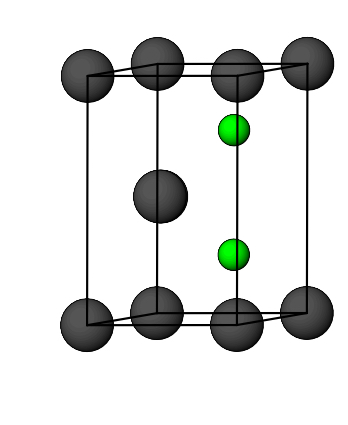
what is the number of octahedral holes per unit cell for HCP
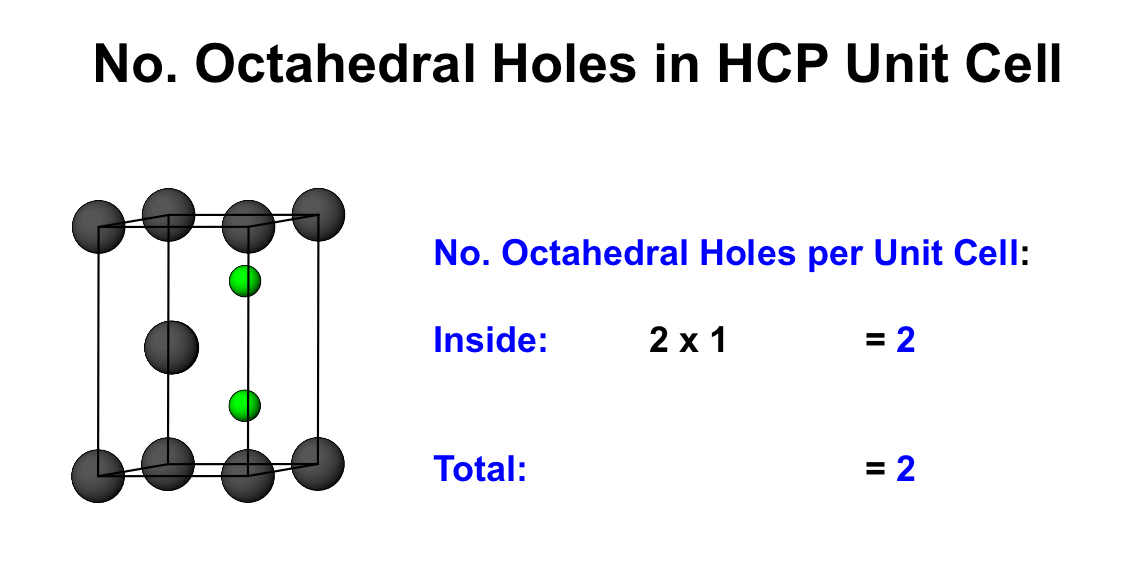
what is the number of anions in a HCP unit cell?
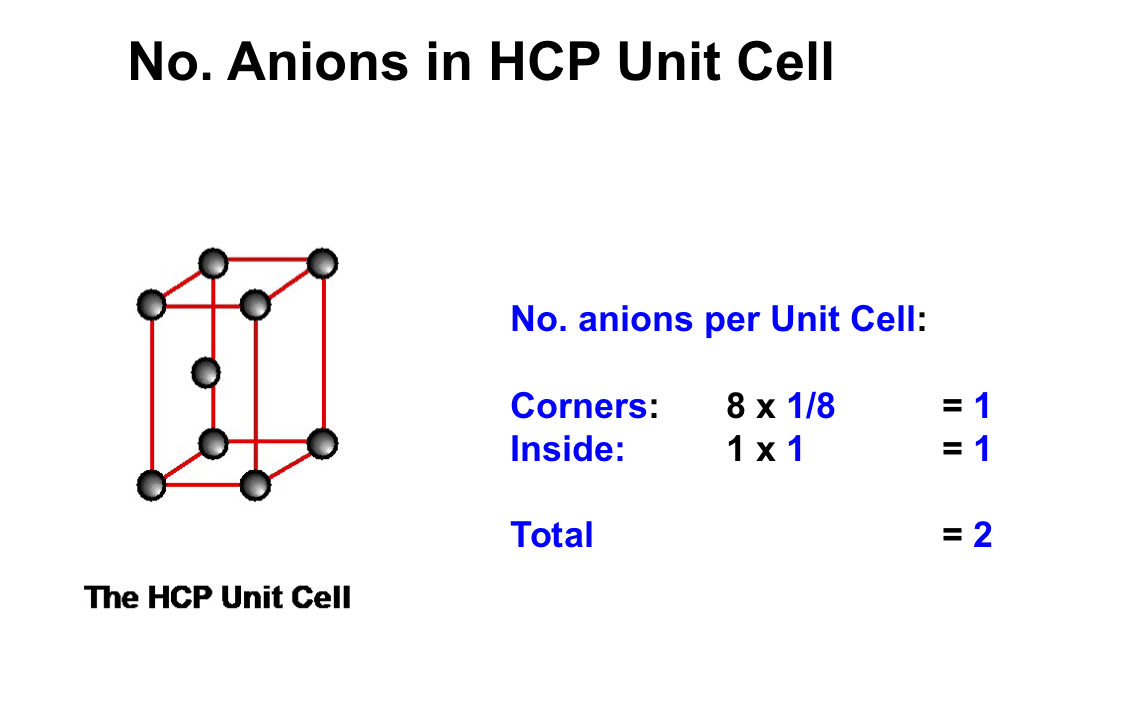
how would you make the number of anions and no. of test holes in a HCP unit cell an AX structure?
half the number of tet holes filled to make it 1:1
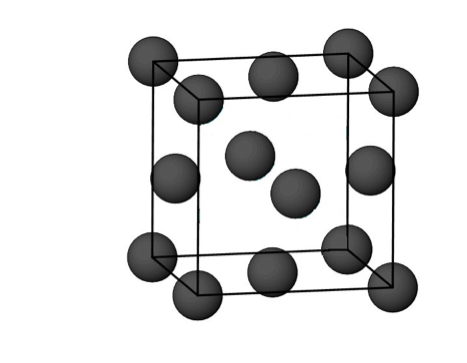
what is the number of anions in a CCP unit cell

what is the number of tetrahedral holes in a CCP structure
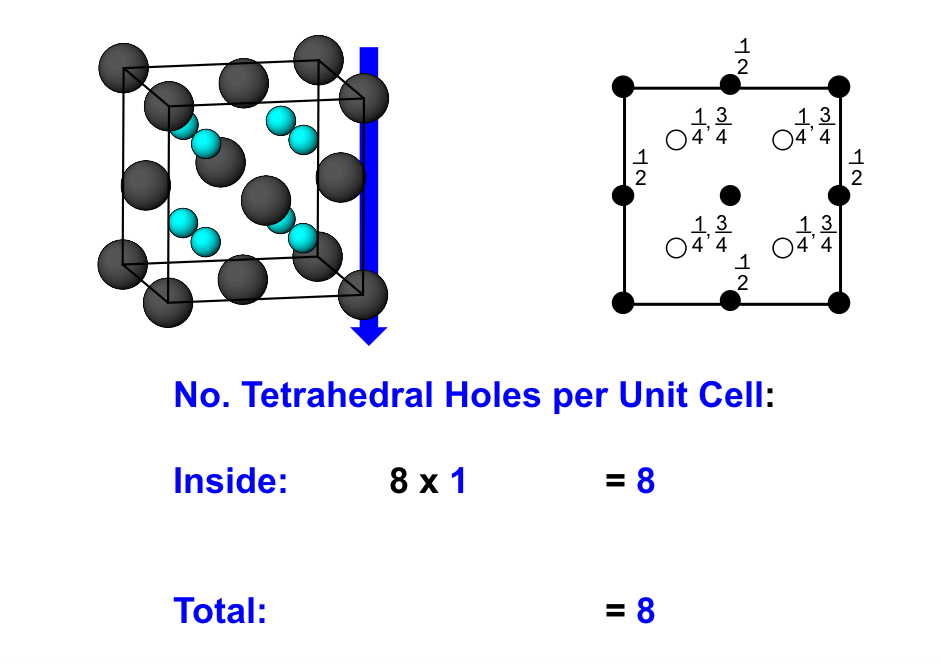
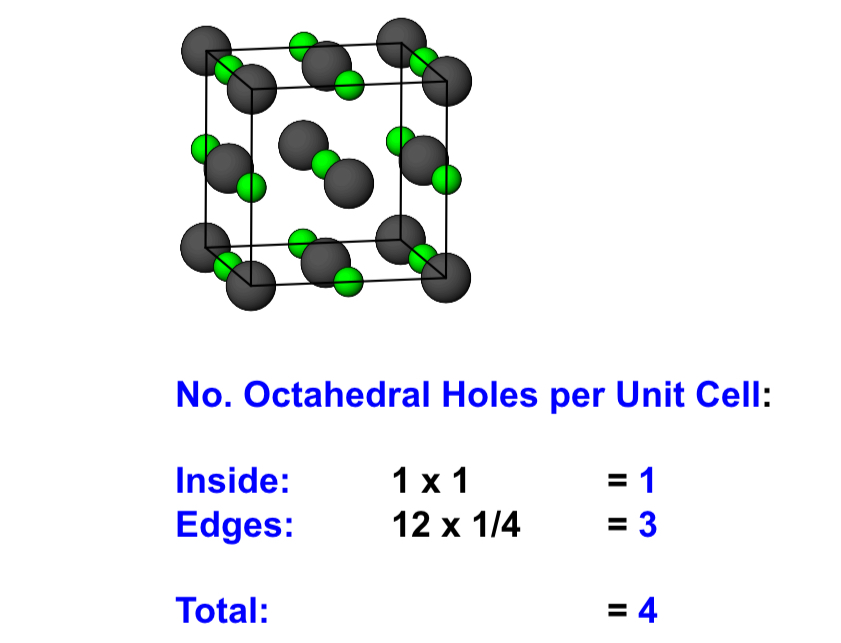
what is the number of octahedral holes in a CCP structure?
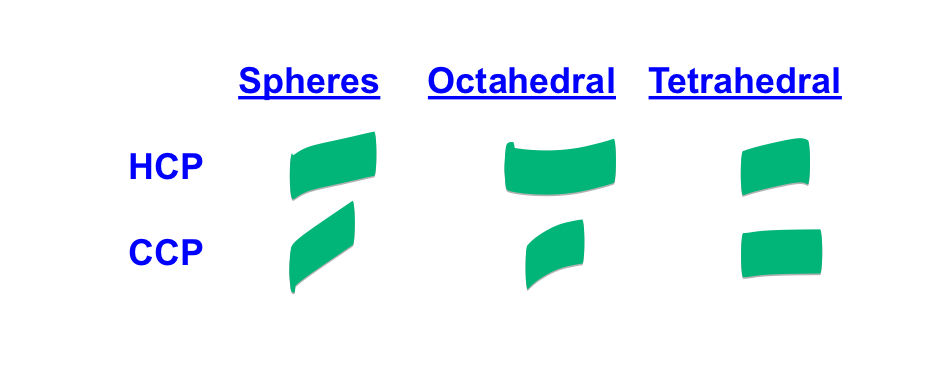
fill this table in
what are the main 5 AX structures?

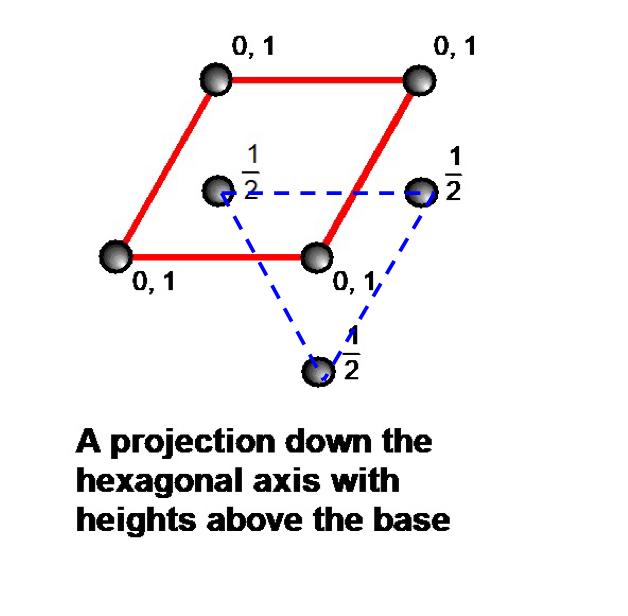
what do tetrahedral holes in HCP structures look like from a projection down the hexagonal axis with heights above the base ? ( include coordinates )
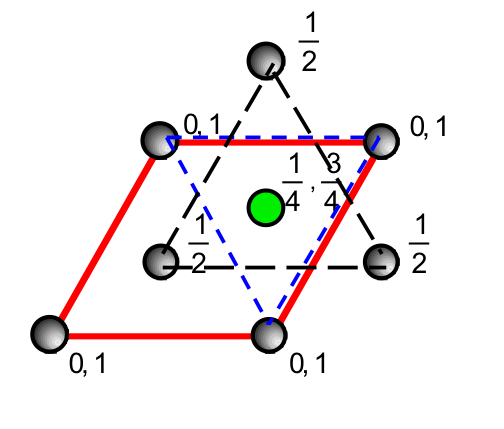
what do octahedral holes in HCP structures look like from a projection down the hexagonal axis with heights above the base ? ( include coordinates )
what is the radius ratio?


fill in this table
why are only half of tetrahedral sites filled for zinc blende structures ?
to minimise cation-cation repulsion
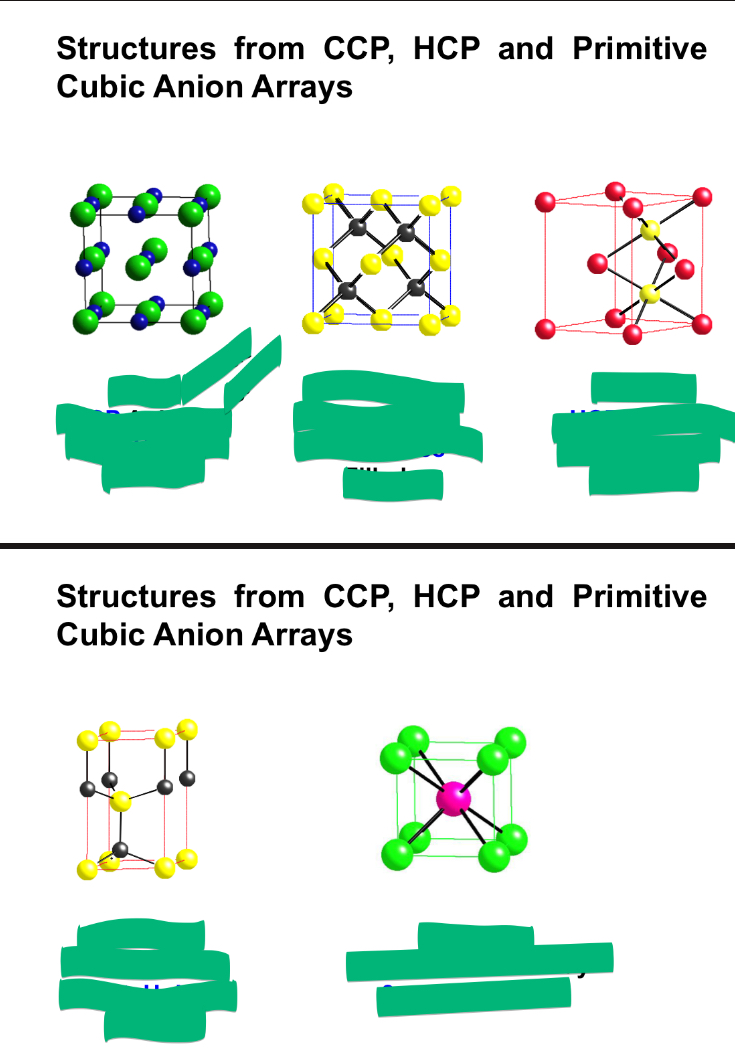
fill this in
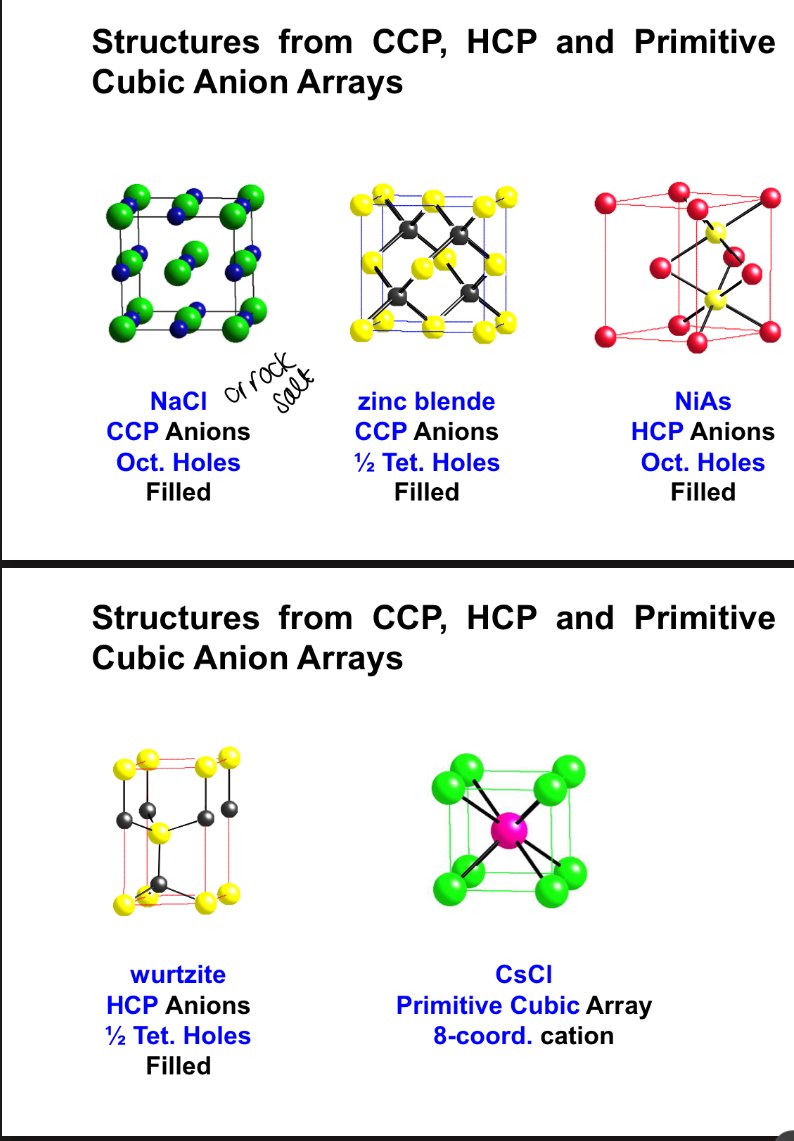
how is the unit cell for a NiAd cell distorted from an ideal HCP lattice? and why ?
by a comparesion along the hexagonal packing axis
due to cov bonding
why are only half the tetrahedral sites filled in a Wurtzite structure?
to minimise cation-cation repulsion’s
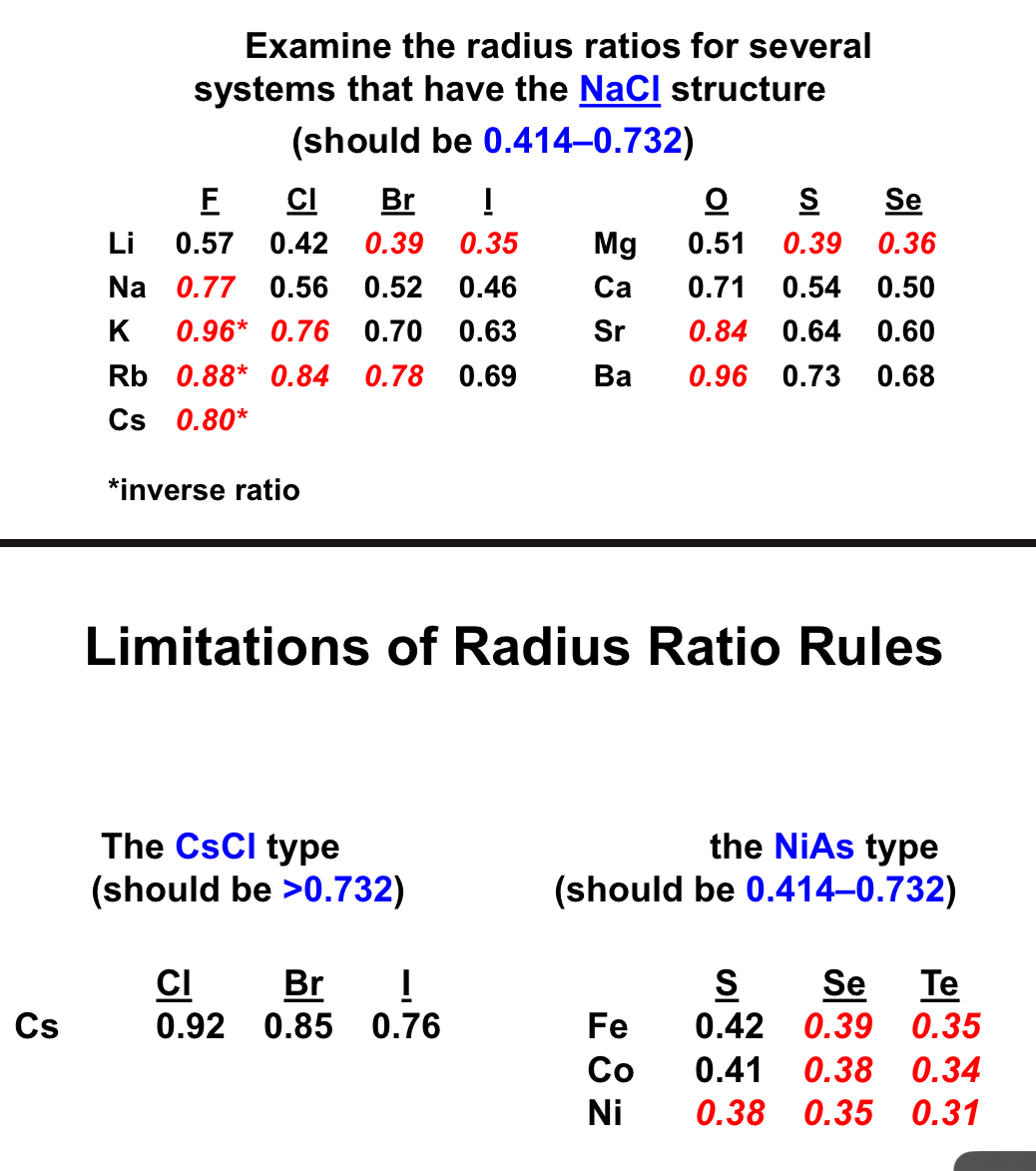
are there limitations of radius rules and give examples ?
yes
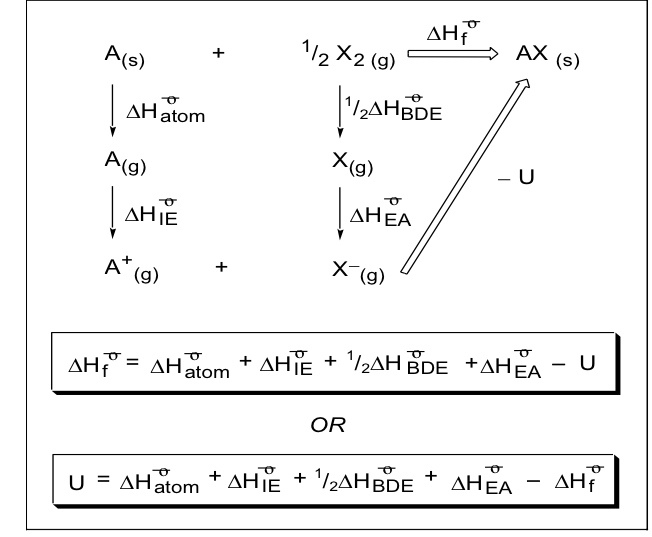
what is the definition for the lattice enthalpy (U) and give the equation ?
the amount of energy required to break up a soldi and form gaseous ions
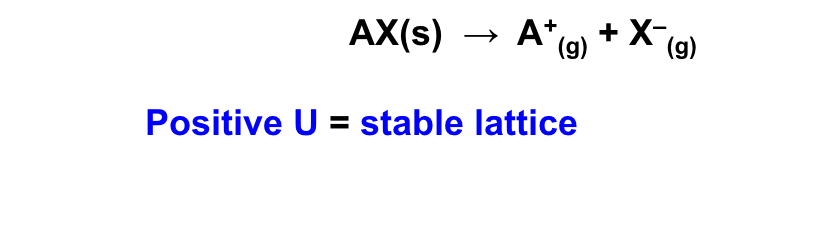
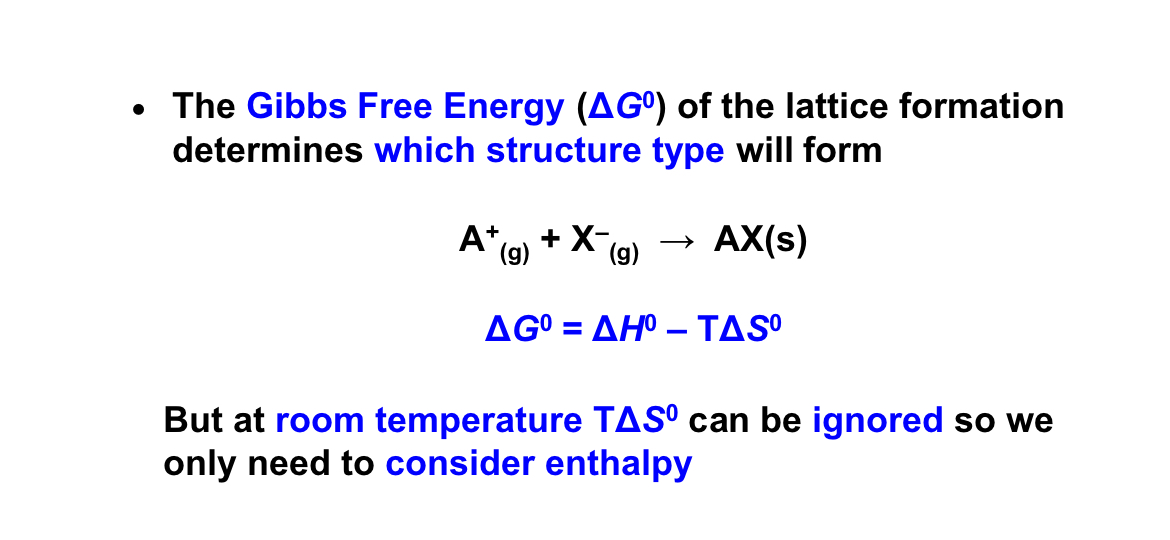
what does the gibbs free energy of the lattice formation determine
what is the born -hater cycle for forming Ax from A(s) + X_2 (g)
what is hess’s law
the energy change of a process depends only upon the energies of the final and initial c states and not the route taken


what is the madelung constant ( A)
the geometric sum of all cation-anion interactions
how to calculate E total ?
E attractive + E repulsive
what is the born Lande equation ( don’t need to memorise it just be aware of it )
us the average value of n for two element
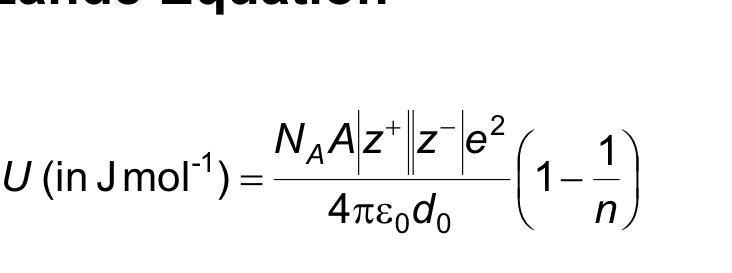
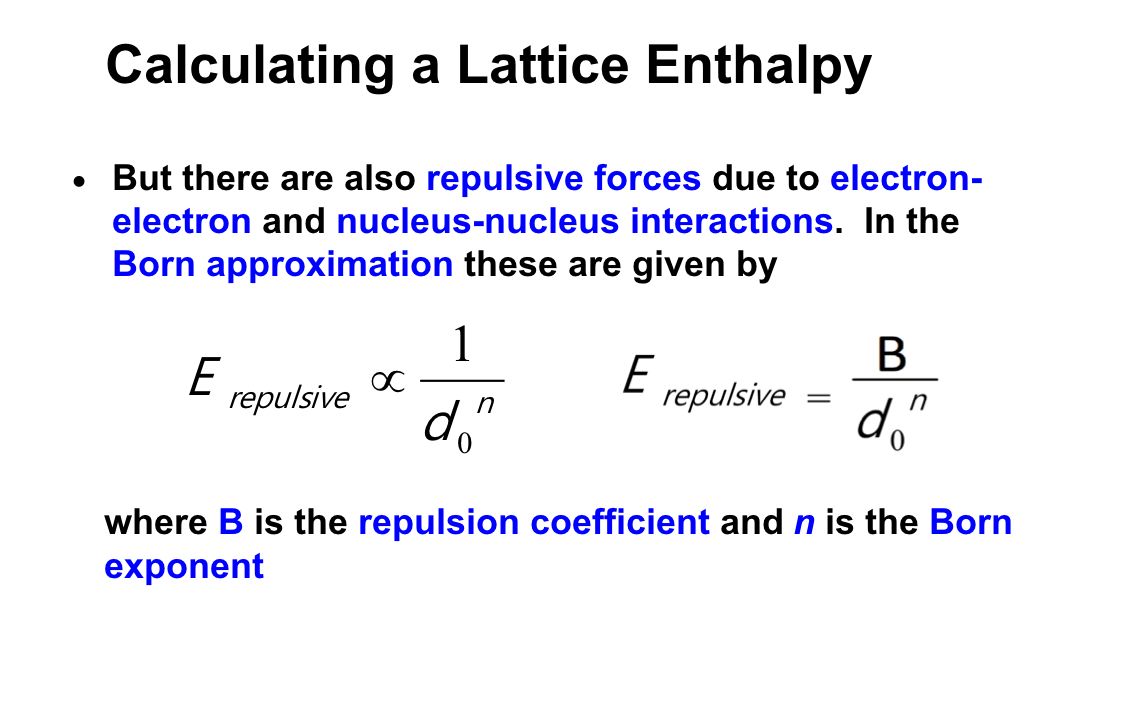
what equation on lines the repulsive forces due to electron and nucleus-nucleus interactions in the born approximation
what are the non-ionic factors in solid affecting the difference in U calculated vs from thermochemical data?
metal-metal bonding
most NiAs structures distort to reduce M-M distance ( transition metal ions ) due to d-d overlap affecting distance
covalency effects :
cov bonding favours lower cordination numbers = shorter anion distances ∴ better orbital overlap
tetrahedral coordation : sp3 hybridisation
zinc blende prefers cation-anion pairs that cov bond despite radius ratio oct prediction
this is seen in crystal field effects => forming square planar