8. Isomerism & Chirality
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What are the two types of isomerism and their sub categories?
Structural isomerism - where the actual bonds are different - linkage isomerism, ionisation isomerism, solvate isomerism, coordination isomerism
Stereoisomerism - molecules with the same molecular and structural formula, but differ in how they are arranged in 3D - geometrical isomerism and optical isomerism
What is linkage isomerism?
Structural isomerism
Ligands may be able to bond through a different donor atom (ambidentate ligands)
For example, NO2- can be bonded through the N (nitro) or one of the Os (nitrito)
The same with M-SCN, bonded through S is thiocyanato, or bonded through N is isothiocyanato
Similar with CN-, bonded through C is cyano, N is isocyano
What is symbiosis?
In linkage isomerism with ambidentate ligands, the harder/softer donor atom will bond depending on the hardness/softness of the central metal ion
A hard ligand can also increase the hardness of the metal ion and attractive more hard ligands and vice versa with a soft ligand.
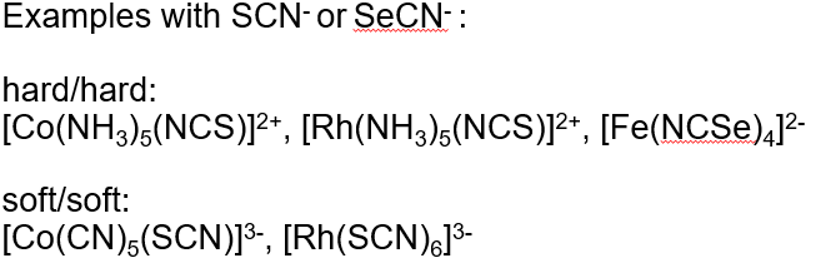
What is ionisation isomerism?
Structural isomerism
Compounds that contain the same atoms but will react differently depending on which ion is actually acting as a ligand and what is outside the square bracket as just a counter ion. - only things inside teh square bracket are covalently bonded
For example, [CoBr(NH3)5]SO4 or [CoSO4(NH3)5]Br
In one case, Br is the counter ion and this can produce a salt when reacted with silver, however is SO4 was outside the square bracket as a counter ion, it would not react with silver to form a salt.
![<ul><li><p>Structural isomerism </p></li><li><p>Compounds that contain the same atoms but will react differently depending on which ion is actually acting as a ligand and what is outside the square bracket as just a counter ion. - only things inside teh square bracket are covalently bonded</p></li><li><p>For example, [CoBr(NH<sub>3</sub>)<sub>5</sub>]SO<sub>4 </sub>or [CoSO<sub>4</sub>(NH<sub>3</sub>)<sub>5</sub>]Br</p></li><li><p>In one case, Br is the counter ion and this can produce a salt when reacted with silver, however is SO4 was outside the square bracket as a counter ion, it would not react with silver to form a salt.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/5a2e78a8-29bb-47d1-8714-07a4135ba75f.png)
What is Solvate isomerism?
Structural isomerism
A special case of ionisation isomerism where there are neutral solvate molecules - H2O
Can also be called hydrate isomerism
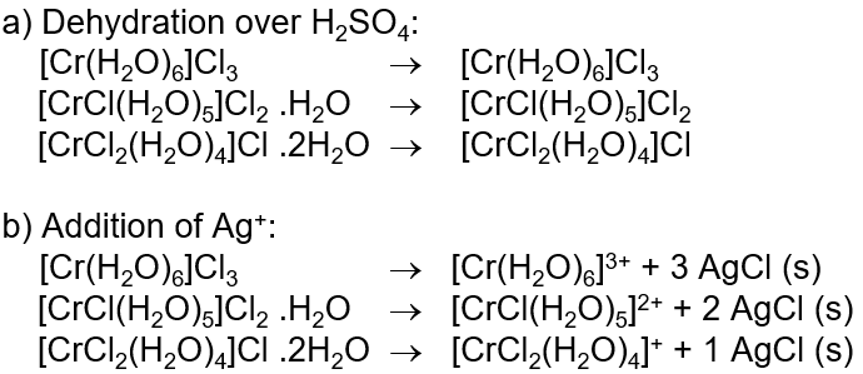
What is coordination isomerism?
Structural isomerism
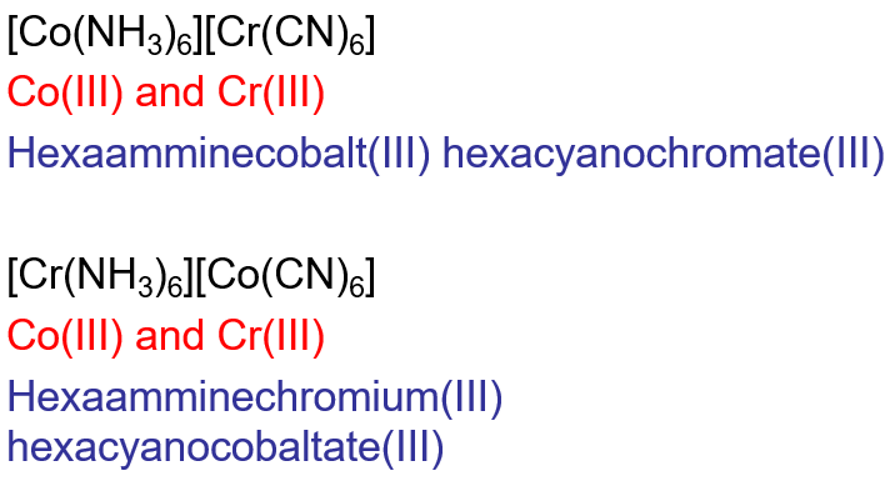
Where ligands may interchange between cationic and anionic complexes
A joint double salt can have two complexes where the metals swap ligands and the overall empirical formula would be identical but the complexes within it are different.
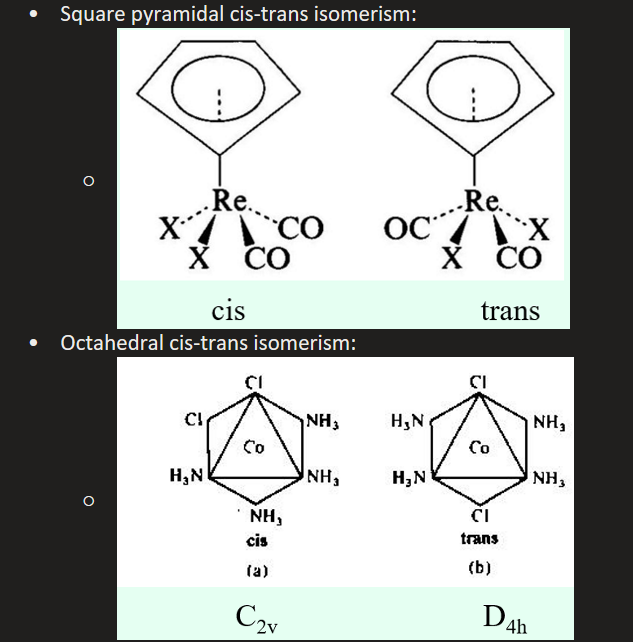
What is cis/trans geometrical isomerism? What geometries can this occur under?
Stereoisomerism
The occurrence of cis and trans complexes
Does NOT occur in tetrahedral complexes
Can be seen in square planar, square pyramidal and octahedral if it has MA2B4 formula
For octahedral, both of the A2 atoms will be axial to be trans - cis-trans isomerism within octahedral molecules can cause colour changes in the complex
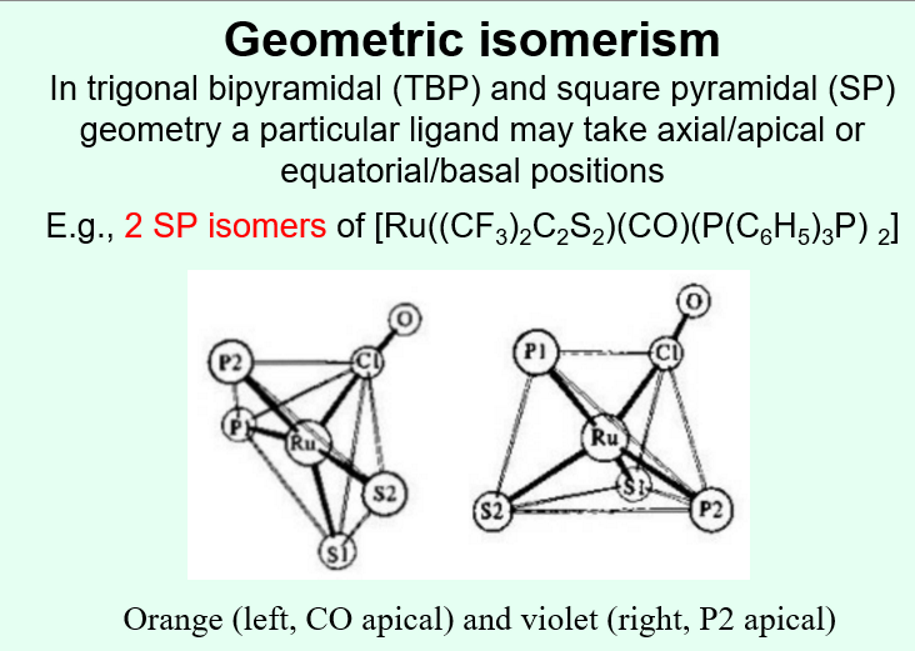
What form of geometric isomerism can be seen in trigonal bipyramidal and square planar complexes?
axial/apical or equatorial/basal position isomerism
particular ligands may take the axial or equatorial positions and this can in some cases change the colour of the complex.
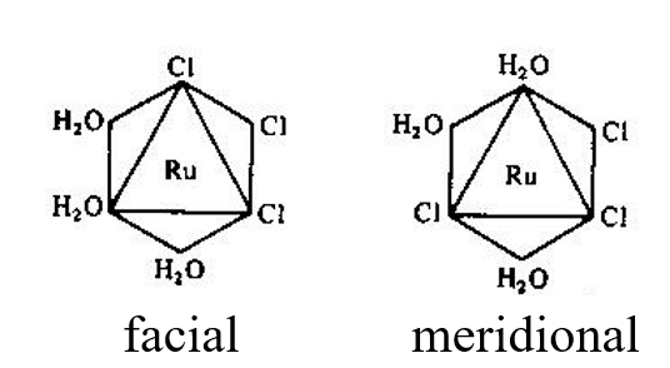
What layout of ligands can occur for a MA3B3 octahedral complex?
They can be facial or meridional
Facial is when the same three ligands take up the corners of a triangle to make a ‘face’ of the triangle
Meridional is when three ligands for a line from axial to equatorial to axial
What is optical isomerism? How can it arise in tetrahedral and octahedral complexes?
stereoisomerism
Molecules with the same molecular and structural formula but different arrangements
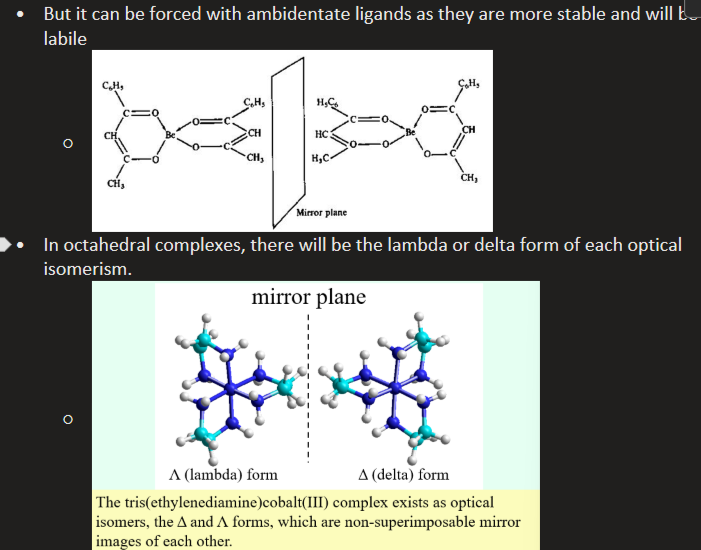
Tetrahedral complexes rarely show chirality due to high liability of tetrahedral complexes leading to fast racemisation - but it can be forced with ambidentate ligands as they are more stable and less liable
In octahedral complexes, there is lambda or delta forms of each optical isomer