4.Properties of Solutions
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What is Adhesion
💧 What Is Adhesion? Definition:
Adhesion is the attraction between molecules of different substances.
In Water:
Water molecules also stick to other materials, especially if they are polar or charged (like glass, soil, or plant cell walls).
🧪 Example of Adhesion:
Water climbing up the walls of a glass tube (meniscus formation).
Water sticking to plant cell walls during capillary action.
What is Cohesion
💧 What Is Cohesion? Definition:
Cohesion is the attraction between molecules of the same substance.
In Water:
Water molecules are polar, meaning they have a slightly positive side (hydrogen) and a slightly negative side (oxygen). This polarity causes water molecules to stick to each other through hydrogen bonds.
🧪 Example of Cohesion:
Water droplets are forming on a surface.
Surface tension: Water striders can walk on water due to water's cohesive forces.
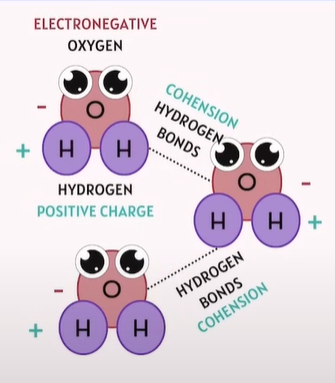
Polarity of Water
🔗 How Do Water Molecules Combine with Each Other? Via Hydrogen Bonding:
The oxygen atom (slightly negative) of one water molecule attracts the hydrogen atom (slightly positive) of another water molecule.
This hydrogen bond is not as strong as a covalent bond, but it's strong enough to create cohesion.
💡 TEAS Exam Insight:
This bonding explains:
Why water has high surface tension
Why it resists temperature change (high specific heat)
Why it can move upward in plants (capillary action)
🧠 Flashcard Questions
Front | Back |
|---|---|
What kind of bond allows water molecules to stick together? | Hydrogen bonds. |
Give an example of cohesion in water. | Surface tension causes water droplets to form. |
Give an example of adhesion in water. | Meniscus in a glass tube or water climbing up plant roots. |
Solute, Solvent & Solution
What is the universal solvent?
🧪 Key Terms Explained
Term | Definition | Example (Salt Water) |
|---|---|---|
Solute | The substance that is being dissolved in a solution. | Salt (NaCl) |
Solvent | The substance that does the dissolving — typically present in the greatest amount. | Water |
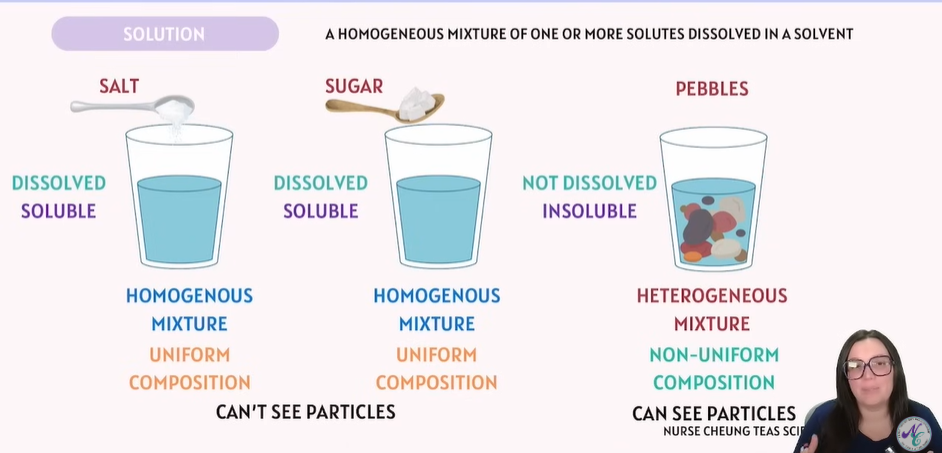
Solution | A homogeneous mixture of a solute dissolved in a solvent. | Salt water (salt + water) |
What is the universal solvent? - Water
🧠 Quick Memory Tip:
"Solute gets dissolved, solvent does the dissolving → together they make a solution."
🧠 TEAS Flashcard Questions
Front | Back |
|---|---|
What is a solute? | The substance being dissolved (e.g., salt or sugar). |
What is a solvent? | The substance doing the dissolving (e.g., water). |
What is a solution? | A homogeneous mixture of solute and solvent. |
In a sugar water mixture, what is the solute? | Sugar. |
In a sugar water mixture, what is the solvent? | Water. |
What kind of mixture is a solution: homogeneous or heterogeneous? | Homogeneous — the composition is uniform. |
Hydrophilic & Hydrophobic
💧 Key Concepts from the Diagram
Property | Polar Substances | Non-Polar Substances |
|---|---|---|
Solubility in Water | ✅ Soluble in water | ❌ Insoluble in water |
Examples | Salt, sugar | Oil, fat |
Water Affinity | Hydrophilic (water-loving) | Hydrophobic (water-fearing) |
Polarity | Have partial charges (dipoles) | No charge separation |
🧪 Definitions 🔹 Hydrophilic
Means “water-loving”
These are usually polar substances that dissolve well in water.
Examples: Salt (NaCl), sugar (C₆H₁₂O₆)
🔸 Hydrophobic
Means “water-fearing”
These are non-polar substances that do not dissolve in water.
Examples: Oils, fats, waxes
Molarity & Dilution & Concentration
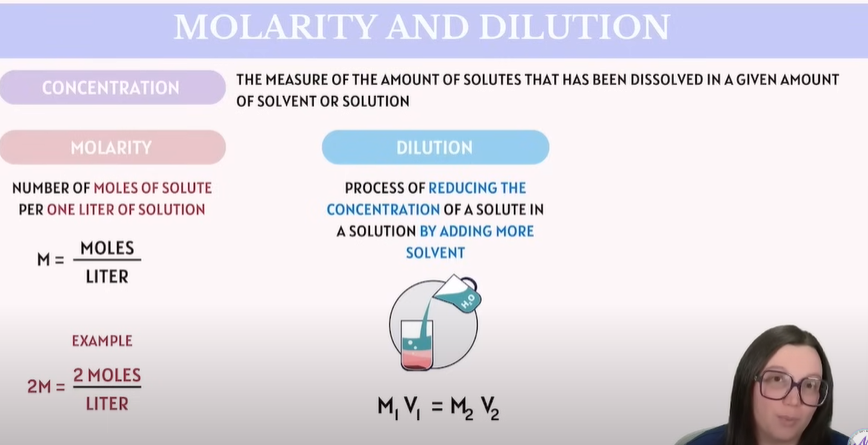
⚗ 1. Molarity (M) 🔹 Definition:
Molarity is a measure of concentration — it tells you how many moles of solute are in 1 liter of solution.
📌 Formula: Molarity (M)=moles of soluteliters of solution\text{Molarity (M)} = \frac{\text{moles of solute}}{\text{liters of solution}}Molarity (M)=liters of solutionmoles of solute 🧪 Example:
If you dissolve 2 moles of NaCl in 1 liter of water, the molarity is:
M=2 moles1 L=2 MM = \frac{2 \text{ moles}}{1 \text{ L}} = 2\,MM=1 L2 moles=2M
🧴 2. Concentration 🔹 Definition:
Concentration refers to how much solute is present in a given amount of solvent or solution.
It can be expressed in different ways:
Molarity (mol/L) – most common on TEAS
Percent concentration (e.g., 5% NaCl)
Grams per liter (g/L)
🧠 TEAS Tip:
When the exam says something like “a concentrated or dilute solution,” they’re referring to how much solute is dissolved:
High concentration = lots of solute
Low concentration = little solute
💧 3. Dilution 🔹 Definition:
Dilution means adding more solvent (usually water) to a solution, which lowers its concentration but keeps the amount of solute the same.
📌 Formula: M1V1=M2V2M_1V_1 = M_2V_2M1V1=M2V2
Where:
M1M_1M1 = initial molarity
V1V_1V1 = initial volume
M2M_2M2 = final molarity
V2V_2V2 = final volume
🧪 Example:
You have 100 mL of 2 M HCl and want to dilute it to 1 M. How much total solution will you have?
(2)(100)=(1)(V2)⇒V2=200 mL(2)(100) = (1)(V_2) \Rightarrow V_2 = 200\,mL(2)(100)=(1)(V2)⇒V2=200mL
So, you’d add 100 mL of water to make it 200 mL total.
🧠 Flashcard Review
Front | Back |
|---|---|
What is the formula for molarity? | M = moles of solute / liters of solution |
What happens to concentration when you dilute a solution? | It decreases. |
What does the equation M₁V₁ = M₂V₂ represent? | The dilution formula |
If you add water to a solution, what changes? | Volume increases, concentration decreases (solute amount stays same). |
What is concentration? | The amount of solute per volume of solution. |
🧪 TEAS Exam Tips:
✅ Expect questions like:
“What is the molarity if 0.5 moles are dissolved in 250 mL?”
“How much water must be added to dilute a 3 M solution to 1 M?”
“Which of these solutions is more concentrated?”

Molarity & Dilution & Concentration - Practice

Osmosis
🔹 Osmosis
✅ Definition:
A type of diffusion involving water molecules moving through a semipermeable membrane from an area of low solute concentration to high solute concentration.
✅ Key Points:
Only for water movement.
Moves to balance solute concentration on both sides.
Example: Water moving into root hair cells of plants; water reabsorption in kidneys.
Tips:
Think of H2O “O” in Osmosis = Movement of water
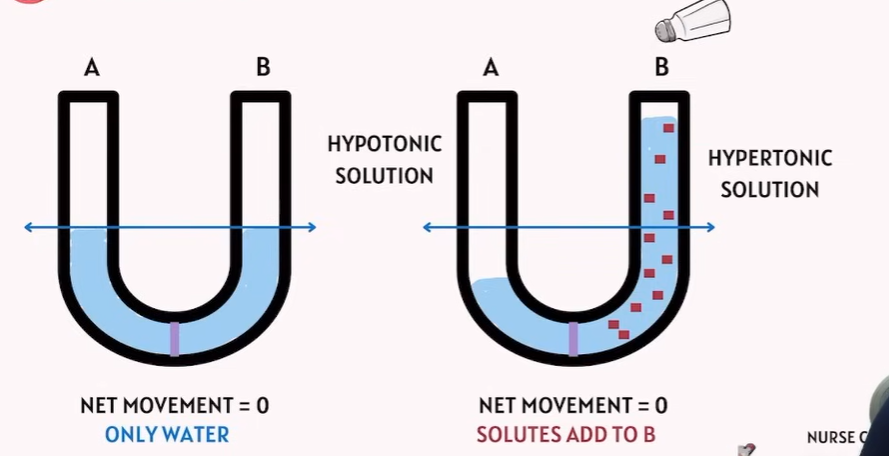
Hypotonic and Hypertonic ? how Solutes play a role in there?
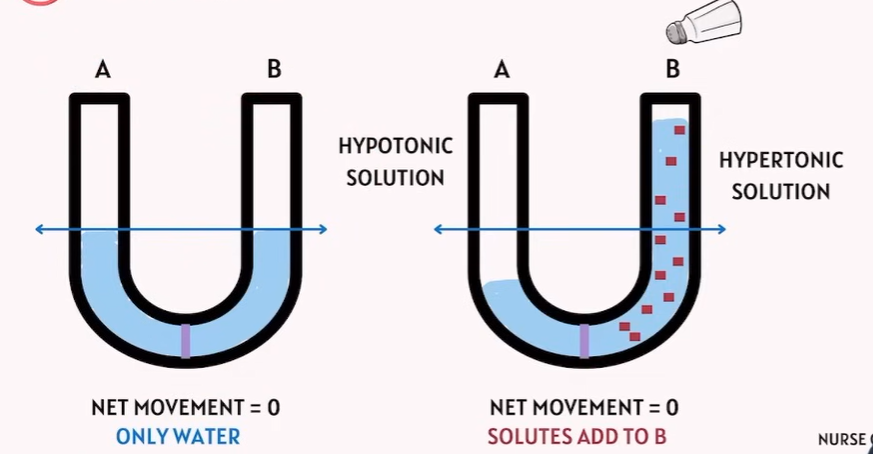
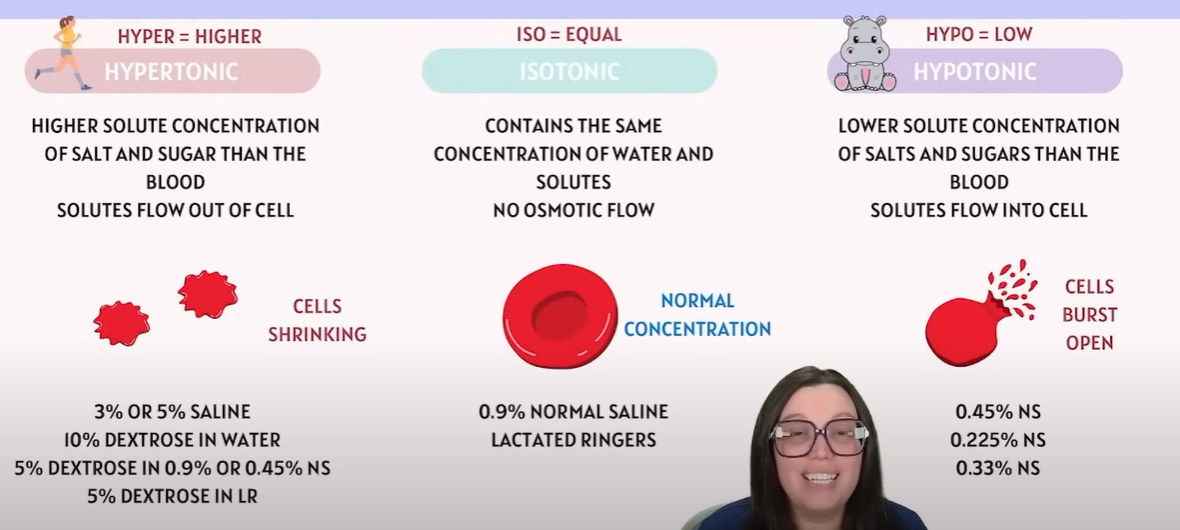
🔹 Key Definitions
Term | Meaning | Effect on cell |
|---|---|---|
Hypotonic solution | Lower solute concentration outside the cell compared to inside | Water moves into the cell → Cell swells or bursts (lysis) |
Hypertonic solution | Higher solute concentration outside the cell compared to inside | Water moves out of the cell → Cell shrinks (crenation) |
Isotonic solution | Same solute concentration inside and outside | No net water movement → Cell stays the same size |
Diffusion
Net movement (Overall Movement) of anything generally from a region of higher concentration to a region of lower concentration driven by a gradient in free energy or chemical potential. Passive Transport
Note: Osmosis talks about water, but Diffusion talks about movement of particles, not water.
🔹 Key Characteristics
✔ Passive process – does not require energy (ATP).
✔ Occurs because of random movement of particles.
✔ Continues until equilibrium is reached (equal concentration everywhere).
✔ Can happen in air, liquid, or across membranes.
🔹 Examples in the Body
Oxygen diffusing from alveoli (lungs) into blood capillaries.
Carbon dioxide diffusing from blood into alveoli to be exhaled.
Perfume scent spreading across a room.
🔑 Quick Summary
✔ Diffusion = high → low concentration without energy.
Facilitated Diffusion & Protein Channels
🔹 Facilitated Diffusion
✅ Definition:
Facilitated diffusion is the movement of molecules across a cell membrane via transport proteins from high concentration to low concentration.
✅ Key Points:
Passive transport – does not require energy (ATP).
Helps large or charged molecules (like glucose, ions) cross the membrane, which cannot pass directly through the lipid bilayer.
🔹 Protein Channels
✅ What are they?
Transmembrane proteins that form pores or channels in the membrane.
Allow specific molecules or ions to pass through by facilitated diffusion.
✅ Examples:
Ion channels – for Na⁺, K⁺, Ca²⁺, Cl⁻.
Aquaporins – special channels for water transport.
🔑 Quick Comparison:
Simple Diffusion | Facilitated Diffusion |
|---|---|
Molecules pass directly through membrane | Molecules pass through protein channels or carriers |
For small, nonpolar molecules (O₂, CO₂) | For large, polar, or charged molecules (glucose, ions) |
No protein needed | Requires transport protein |
Passive | Passive |
Factors Affecting Diffusion(4)
🌟 Factors Affecting Diffusion – Table
Factor | Description | Effect |
|---|---|---|
Distance | The distance particles travel. | Greater distance = slower diffusion rate. |
Temperature | Heat energy affecting particles. | Higher temperature = faster diffusion rate. |
Solvent Characteristics | Density of the medium. | Increased density (thicker fluid) slows diffusion. |
Traveling Characteristics (Mass) | Mass of the diffusing particles. | Greater mass = lower diffusion rate. |
Barrier Characteristics | Membrane permeability and cell polarity. | Small, non-polar molecules pass through barriers more easily. (Note from bottom text) |
✨ Flashcard Questions
Question | Answer |
|---|---|
How does distance affect diffusion rate? | Greater distance slows diffusion. |
How does temperature affect diffusion rate? | Higher temperature increases diffusion rate. |
How does solvent density affect diffusion rate? | Higher density slows diffusion rate. |
How does particle mass affect diffusion rate? | Greater mass decreases diffusion rate. |
What type of molecules pass through barriers easier? | Small, non-polar molecules. |
Summary Tip
📝 TEAS Key:
“Diffusion is faster with high temperature, small particles, short distance, low solvent density, high concentration gradient, and large surface area.”
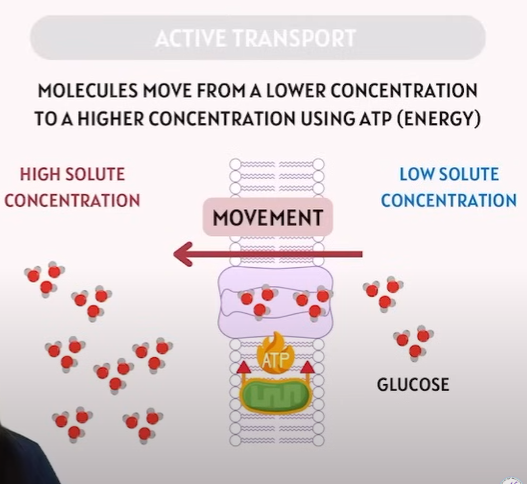
Active Transport
🌟 Active Transport – Explanation
Feature | Explanation |
|---|---|
Definition | Movement of molecules from low concentration to high concentration (against the gradient). |
Energy? | Requires ATP energy. |
Direction? | Moves against the concentration gradient (opposite of diffusion). |
Example in Image | Glucose being transported into a cell using a carrier protein and ATP. |
Key Protein Type | Carrier proteins or pumps (e.g. sodium-potassium pump). |
✨ Flashcard Questions
Question | Answer |
|---|---|
What is active transport? | Movement of molecules from low to high concentration using energy. |
Does active transport require energy? | Yes, it uses ATP. |
Which direction does active transport move substances? | Against the concentration gradient. |
What is an example of active transport? | Sodium-potassium pump; glucose uptake in intestines. |
What type of proteins are involved in active transport? | Carrier proteins or pumps. |
🔑 Extra Exam Points Missing From Image
✔ Types of Active Transport:
Primary Active Transport:
Direct use of ATP (e.g. sodium-potassium pump).
Secondary Active Transport (Cotransport):
Uses energy indirectly by coupling with another molecule moving down its gradient (e.g. glucose-sodium symport).
✔ Sodium-Potassium Pump:
Pumps 3 Na⁺ out and 2 K⁺ into the cell.
Maintains resting membrane potential.
📝 TEAS Tip
🔹 Active transport = ATP needed + low → high concentration.
🔹 Diffusion/facilitated diffusion = no ATP + high → low concentration.
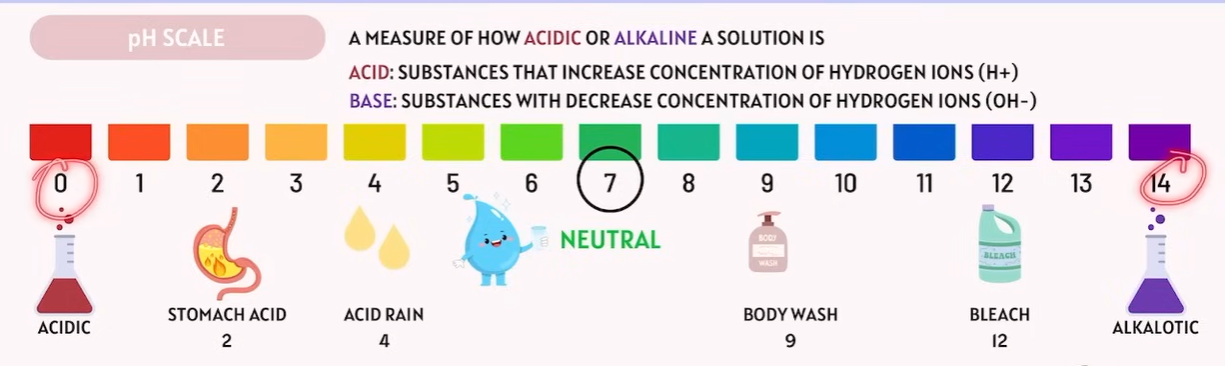
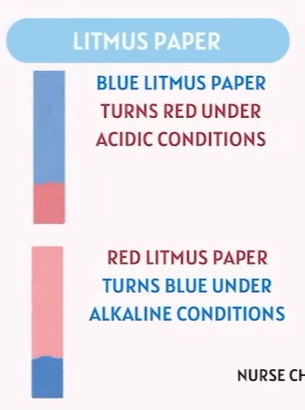
Acids and Bases
📝 TEAS Tip
Remember:
Acids → donate H⁺ ions.
Bases → accept H⁺ ions or donate OH⁻ ions.
Acid: Higher Hydrogen ion concentrations make the solution more acidic.
Alkaline: Less Hydrogen ion concentrations make the solution more Alkaline.Lower hydrogen
Neutralization Reaction
Acids
Memory Tip: Acid names often end in “-ic”
Common Acids:
Hydrochloric Acid → HCl
Sulfuric Acid → H₂SO₄
Nitric Acid → HNO₃
Bases
Memory Tip: Base names often end in “-oxide” or “-nate”
Common Bases:
Sodium Hydroxide → NaOH
Calcium Carbonate → CaCO₃