A1.1 + A1.2
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
72 Terms
State that the first cells originated in water.
Molecular ingredients of life needed to react with each other in a liquid solvent.
List reasons why water is a substance on which life depends
Water is a solvent, metabolite, temperature buffer and maintains biological structures.
Describe the structure of an atom.
An atom has protons, neutrons and electrons.
Outline the formation of a water molecule
A covalent bond joins H and O atoms in a water molecule, involving the exchanging the electrons.
Explain the sharing of electrons between atoms in a polar covalent bond. Within a water molecule
The electrons in the covalent bond are not equally shared as the O atom tends to pull the H electrons closer.
State the location of the polar covalent bond within a water molecule.
The bent shape of a water molecule results i hydrogen atoms being on one side and the oxygen atom being on the other. Resulting in a polar covalent bond.
Explain the partial charges of the oxygen and hydrogen atoms within a water molecule.
A partial negative charge on the Oxygen atoms and a partial positive charge o the Hydrogen atom.
Outline the cause of the formation of hydrogen bonds between water molecules.
The partial charges and dipolarity of water molecule attract other water molecule and weak intermolecular forces called hydrogen bonds form between them.
Outline the consequences of the collective strength of hydrogen bonds between water molecules.
Hydrogen bonds are weak intermoleclar forces, but the vast number of them that form per unit volume of water give water its unique properties, which are important to living organisms.
Define cohesion.
Water’s ability to make hydrogen bonds with itself causes water molecules to stick together, a property called cohesion.
Describe how water moves through the xylem of a vascular plant.
Transpiration occurs because stomata are open to allow gas exchange for photosynthesis. This creates tension, which pulls water upward. Cohesion pulls up water molecules in a chain as the top most water is pulled up and out of the stomata.
Outline the cause of surface tension.
A property of the surface of a liquid that allows it to resist on extreme force, due to cohesive nature of its molecules.
State a benefit to living things that results from surface tension.
Allows organisms like water staider to walk on water and providing a stable environment.
Define adhesion.
The interaction between water and solid surface is known as adhesion.
Explain why water is attracted to surfaces that are polar or charged.
Water is atracted to porus solids such as papr. This is due to paper’s large surface area attraction to water.
Outline the cause of capillary action.
When water interacts with a solid surface, adhesion allows it to stick. However when adhesion causes movement, it is called the capillary action.
Describe capillary action in plant tissue.
The capillary action provides rewetting of the dired cell wall due to cellulose molecules.
Outline the cause and effect of capillary action in soil.
If the soil is porus, using the capillary action, water is drawn through dry soil. Water is able to rise up from an underground source even though gravity is pulling it down.
Define solvation
Solvation is when a solute dissolves in a solvent to make a solution.
Explain why water is able to dissolve charged and polar molecules.
polar and ionic molecules are hydrophilic because water’s partial charges are chemically attracted.
Outline the solvation of hydrophilic and hydrophobic substances.
Hydrophilic substances love water, while hydrophobic substances repel water.
State an example of the function of a molecule depending on it being hydrophobic and insoluble.
Sulfur dioxide is a non-polar molecule and are hydrophobic because they have no full or partial charges to attract water.
State an example of the function of a molecule depending on it being hydrophilic and soluble.
Glucose has many polar covalent bonds between Oxygen and hydrogen resulting in partial poles. Due to its covalent bonds, it interacts with water and is dissolved in it.
Outline the role of water as a medium for metabolism.
Water is the medium for metabolism. Cytoplasm of cells are aqueous. Dissolved substances can move around and interact. If substances are dissolved, chemical reactions occur.
Outline the steps of metabolism
(1) Substrate entering active site of enzyme. (2) Enzyme/substrate complex. (3) Products leaving active site of enzyme. (4) Enzyme products complex.
Describe the role of water as a medium for transport in vascular plants.
Xylem transports sap and mineral ions. Phloem transports sucrose and other products of photosynthesis.
Describe the role of water as a medium for transport in animal blood.
Transports are carried in blood plasma. Includes ions, amino acids and glucose. Transporting small amounts of oxygen via red blood cells and hemoglobin binding sites for oxygen. Phospholipids will coat fat molecules in a single layer that allows for them to be transported via blood plasma.
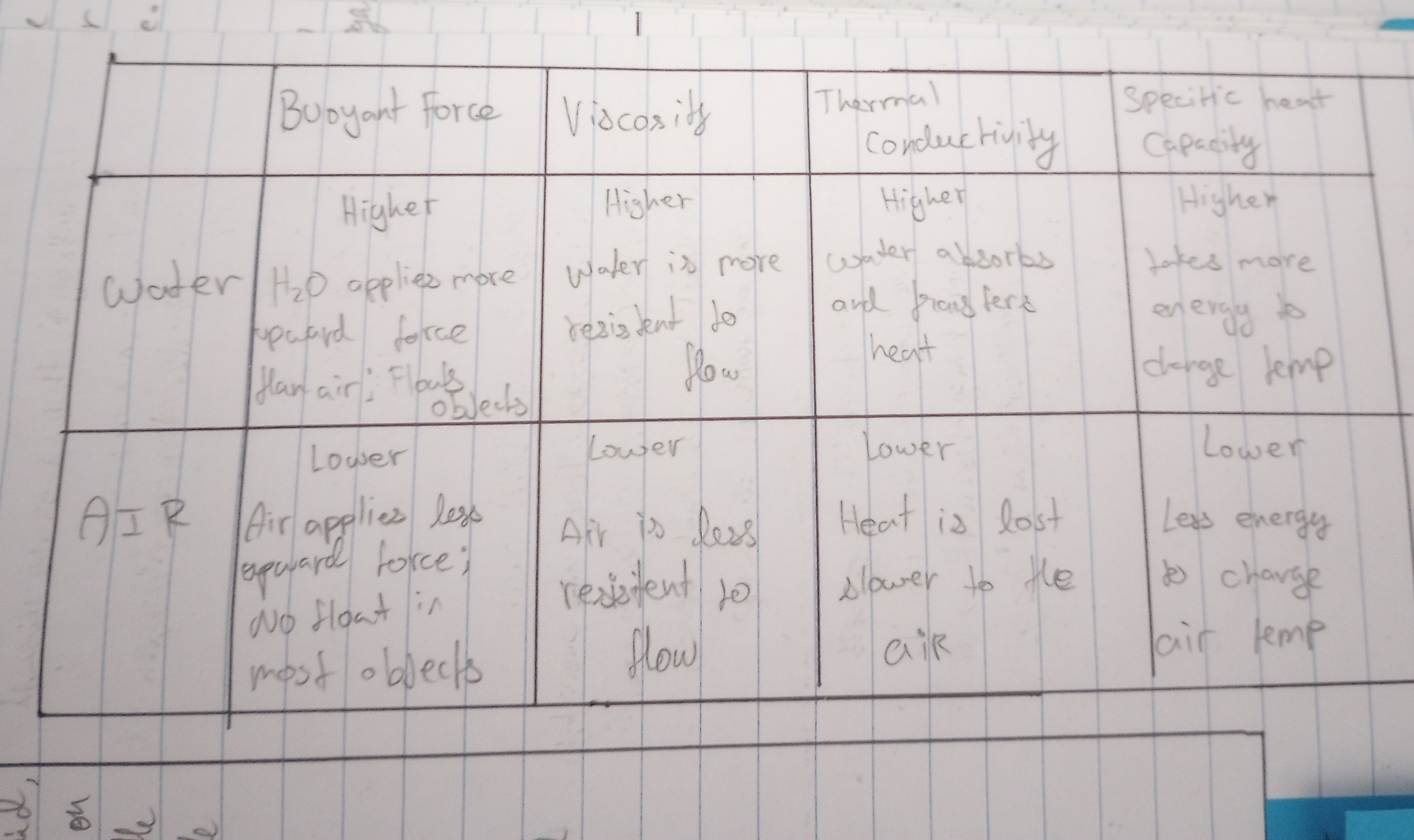
Outline the cause and effect of buoyancy.
The ability or tendency to float in water or air or some other fluid.
Outline the cause and effect of viscosity.
Viscosity is the stickiness of a fluid which determines how easily it can flow.
Compare viscosity of air to water to air.
Water is more resistant to flow than air.
Define thermal conductivity.
The rate at which heat passes through a material is known as thermal conductivity.
Compare less conductive to more conductive materials.
Plastic is less conductive while metal is conductive.
Outline a consequence to life of the thermal conductivity of air and water.
Water absorbs and transfer heat. Where as heat is lost slower to the air.
Define specific heat capacity.
The heat required to raise the temperature of 1g of a material by 1 oC.
Describe why water has a high specific heat capacity.
Water takes more energy to change temperature.
Compare the physical properties of water to those of air.
Describe how the black-throated loon (Gavia arctica) and/or the ringed seal (Pusa hispida) interact with the physical properties of water in their habitat.
Loon spends more energy to stay afloat. Water is more viscous. seal spends more energy moving. Seal’s take more heat, but have a stale thermal environment for the seal.
Explain the hypothesis that asteroids are responsible for the origin of water on Earth.
The most widely supported theroy states that the water arrived on Earth by colliding asteroids.
State two reasons why water was retained on early Earth.
The distance of the earth from the sun 2. Earth’s size and relatively strong gravity.
Explain why the presence of water is considered fundamental to the search for extraterrestrial life.
Liquid water is essential to all known life form on earth.
Define “Goldilocks zone” in relation to the search for extraterrestrial life.
Goldilocks zone is the habitable zone around a star. The zone changes depending on the size of the star and planet. Estimated that 40 billion planets in our galaxy are within this zone.
State the two primary functions of nucleic acids.
Storing of genetic material and duplicating information cell to cell.
State the two types of nucleic acids used in cells.
Deoxyribonucleic acid and ribonucleic acid.
Outline the meaning and implication of DNA being the genetic material of all living organisms.
This is due to DNA storing information of creating new cells.
State why RNA viruses do not falsify the claim that all living things use DNA as the genetic material.
Viruses use RNA as their genetic material, but viruses are not considered to be living.
List the three components of a nucleotide.
Pentose sugar, phosphate group and nitrogenous base.
Define “backbone” as related to nucleic acid structure.
The phosphate of the new nucleotide binds itself to C3 of the previous pentose sugar. Creating a strong sugar-phosphate backbone, conserving the base sequence.
Explain how nucleotides connect to form a nucleic acid polymer.
Covalent bonds are formed between the phosphate of one nucleotide and the pentose sugar of the next nucleotide. This bond i called a phosphodiester bond.
State the names of the nitrogenous bases found in DNA and RNA.
Both DNA and RNA have Adenine, Cytosine and Guanine. However DNA has Thymine while RNA has Uracil.
Outline how the sequence of bases in a nucleic acid serves as a ‘code.’
Any base sequence is possible, making the number of possible sequences almost infinite. The sequence of bases is how information is stored. This coded form is called the genetic code, and is shared by all organisms.
Define gene
A specific sequence of bases in DNA.
Describe the condensation reaction that forms a polymer of RNA from RNA nucleotides.
To make the single-stranded RNA, condensation reactions connect nucleotides. Connecting monomers, producing a hydroxyl molecule. The OH on the phosphate of one nucleotide attaches to the third carbon of the pentose sugar of another nucleotide. One of the OH groups is removed entirely, and hydrogen from the other hydroxyl group is used to produce water.
Identify the monomer and polymer of an RNA molecule.
Nucleotides
Describe the structure of a DNA double helix.
Two strands that are linked by the bases with hydrogen bonds.
Outline the complementary base pairing rule, including the type and number of bonds between bases.
Adenine and Thymine always pairs with 2 hydrogen bonds. Cytosine and Guanine always pairs with 3 hydrogen bonds. This pairing is refereed to as complementary base pairing.
Define antiparallel in relation to DNA structure.
DNA has a helical shape with a constant diameter of 2 nm -creating a double helix. Strands therefore run in opposite directions.
Compare and contrast the structures of DNA and RNA.
DNA is double stranded has ATCG. Where as RNA is single stranded and has ATGU.
Compare and contrast the functions of DNA and RNA.
DNA is used for storing hereditary information, while RNA is used for protein production.
Compare and contrast the location of DNA and RNA in prokaryotic and eukaryotic cells.
DNA is found explicitly in the nucleus. While RNA is made in the nucleus however is transported out via pores. Whereas DNA and RNA are found in the nucleoid in prokaryotic cells.
Outline the role of complementary base pairing in maintaining the DNA sequence during DNA replication.
In DNA replication, the two strands of double helix separate and serve as a template and guide the new starts by adding nucleotides one by one, linking them together. Newly synthesized strands attached to the template strands are the same as the other template strand, known as the semi-conservative model of replication.
Outline the role of complementary base pairing in transmitting the genetic code in transcription and translation.
In this process, a copy of the base sequence is made, but in this case, the copy is an RNA strand, and only one of the two strands is used as a template. This RNA might have a regulatory or structural role, or it may be used in protein synthesis.
Outline why there is a limitless diversity of DNA base sequences.
As the length of DNA strand increases, the number of possibilities become immense. DNA can be any length, the range of possible sequences is effectively limitless, which is an ideal feature for an information storage system.
Define universal in relation to the genetic code.
A codon is a group of 3 bases read together. There are 64 codons, because each base of a codon can by any of four. Most codons specific for a specific amino acid. One codon signal that protein synthesis should start and three codons signal that protein synthesis should stop.
Outline why conservation of the genetic code across all forms of life is evidence of common ancestry.
All living organisms and viruses use the same genetic code - the reason it is called the universal genetic code.
State two benefits of purine-to-pyrimidine bonding on the structure of DNA.
Each pair in DNA has one purine and one pyrimidine base. Leads to the double helix being of equal width, creating a stable structure.
State which bases are purines and pyrimidines
Adenine and Guanine are purine. Where as Cytosine, Thymine and Uracil are pyrimidines.
Compare and contrast the structures of purines and pyrimidines.
Purines have two rings of atoms. Pyrimidines have only one ring of atoms.
Describe the structure of eukaryotic DNA and associated histone proteins during interphase (chromatin).
Eukaryotic DNA is organized in nucleosomes. Each nucleosomes has 8 histone proteins. 2 copies of 4 different types of histone. The DNA then winds approx. twice around this protein. The H1 Histone is an additional protein that reinforced the DNA.
State the experimental question being tested in the Hershey and Chase experiment.
Is genetic material DNA or protein.
Outline the procedure of the Hershey and Chase experiment.
One set of bacteriphages were loaded with radioactive phosphorus. While another set was loaded with radioactive sulfur.
Explain how the results of the Hershey and Chase experiment supported the notion of nucleic acids as the genetic material.
It was noted that radioactive DNA molecules were found in bacteria cells while there were no radioactive protein cells in bacteria cells.
Explain the role of falsifiability in determining the structure and function of DNA.
The idea that in science we can at least be certain of what is not the case, by finding a counter-example.