X-ray Powder Diffraction
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
What do crystalline material produce in XRPD
produce "Bragg peaks"
What do crystalline material produce in XRPD
amorphous materials produce a "halo"
What can XRPD do?
Can easily distinguish between solid forms including polymorphs
Can quantify crystallinity (how much)
What are x-rays?
•Waves (radiomagnetic) with wavelength around 0.1 nm
•X-ray labs often use Cu (copper) as a source of X-rays
o0.154 nm wavelength
•C-C bond length about 0.154 nm
Theory-diffraction
Object > wavelength = shadowing (your hand and light)
Object < wavelength = not visible (atoms and light)
Object similar size to wavelength = diffraction
What is bragg’s law
diffraction happens at angles where
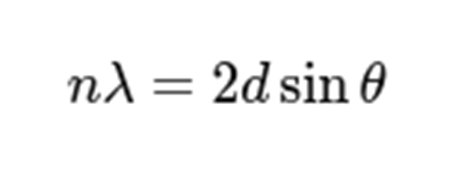
n=1, lambda is wavelength
d is spacing between planes of atoms
theta is (half) the scattering angle
Summary
•Bragg peaks -> crystalline
•Broad "halo" -> amorphous
•Easily distinguishes between different solid forms, including ones with same composition (polymorphs)
•Diffraction – object has similar size to wavelength being used
•Bragg equation - no derivation provided but lots available online
oRelies on constructive vs destructive interference of X-ray waves
XRPD – gold standard for materials analysis of solids
questions
•Why are XRPD patterns unique for each crystalline substance?
•What happens at a diffraction angle where the Bragg equation is not satisfied?
•How does XRPD help in identifying the polymorphic form of a drug?
•If a sample contains 80 % crystalline material and 20 % amorphous, what would the pattern look like?
•Why pattern, why not spectrum?
•How might an increase in temperature affect a XRPD pattern?