Topic 2 - Molecular Biology
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
Define Metabolism
It describes the totality of chemical reactions that occur within a living organism in order to maintain life
Define Catabolism
The breakdown of complex molecules into simpler molecules including the hydrolysis of macromolecules into monomers.
Define Anabolism
The synthesis of complex molecules from simpler molecules including the formation of macromolecules from monomers by condensation reactions
Define Organic Molecules
Chemical substances that contain carbon, but excluding carbonates, hydrogen carbonates and oxides of carbon
Define Cohesion (this of it as ‘coptain‘)
The act, state, or process of sticking together of alike molecules or entities.
Define Adhesion (think of it as apart)
The ability of dissimilar molecules to stick together
Define Capillary Action
A blend of cohesion and adhesion, capillary action sees water moving against gravity through narrow spaces. The adhesive forces between the water and the tube material draw the liquid upwards, while the cohesive forces ensure the column of water remains intact.
Define Transpiration Pull
Transpiration pull or the suction force is the force which aids in drawing the water upward from roots to leaves. In leaves, some amount of water is used for photosynthesis and excess water is released into atmosphere through openings called as stomata.
Define High Specific Heat Capacity
Specific Heat Capacity is the amount of heat required to raise the temperature of 1 gram of something by 1°C, or more generally, the resistance to temperature change. Water has a very high specific heat capacity, so it can absorb a lot of heat with only a small temperature change.
Define High Latent Heat of Vaporization
Hydrogen bonds hold molecules together very tightly, making it difficult to break them apart. The heat applied to effect a change of state at the boiling point is the latent heat of vaporization.
Define Monomers
Monomer is defined as a simple molecule with two or more binding sites through which it forms covalent linkages with other monomer molecules to form the macromolecule. Monomers are thus building blocks of polymers.
Ex. Carbon, hydrogen, oxygen
Define Dimer
When two molecules (monomers) interact with each other.
Define Polymer
Large molecules, are chains or structures constituted of repeating monomer units. The continuous link of monomers through condensation reactions forms these structures.
Ex. Cellulose, Nylon, Vinule
Define Unsaturated Fatty Acid Chain (can be remember by unstraight)
Unsaturated fatty acids have one or more double bonds. Each double bond may be in a cis or trans configuration. In the cis configuration, both hydrogens are on the same side of the hydrocarbon chain. In the trans configuration, the hydrogens are on opposite sides. A cis double bond causes a kink in the chain.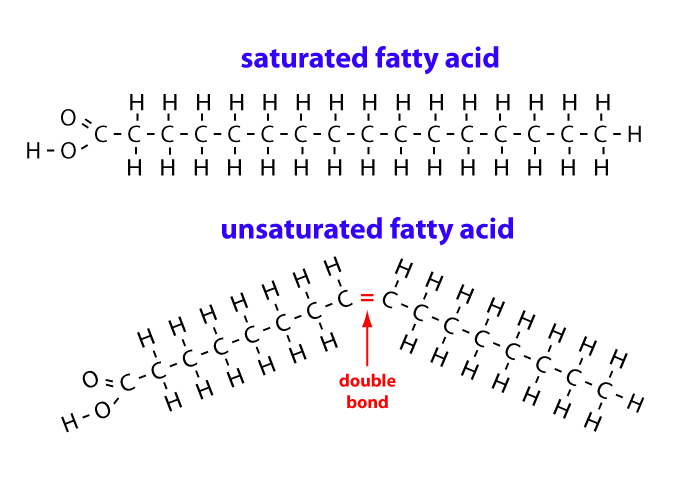
Difference between cis and trans unsaturated fatty acids?
 Can remember it’s related to structure (orientation of Hydrogens)
Can remember it’s related to structure (orientation of Hydrogens)
“Trans”: alternating on both sides
“Cis“: Same side
Define Proteome
The totality of proteins expressed within a cell, tissue or organism at a certain time. The proteome of any given individual will be unique, as protein expression patterns are determined by an individual's genes.
Ex. Enzymes
Define Primary, Secondary, tertiary, and Quaternary Proteins
A protein's primary structure is defined as the amino acid sequence of its polypeptide chain
Secondary structure is the local spatial arrangement of a polypeptide's backbone (main chain) atoms
Tertiary structure refers to the three-dimensional structure of an entire polypeptide chain
Quaternary structure is the three-dimensional arrangement of the subunits in a multisubunit protein.
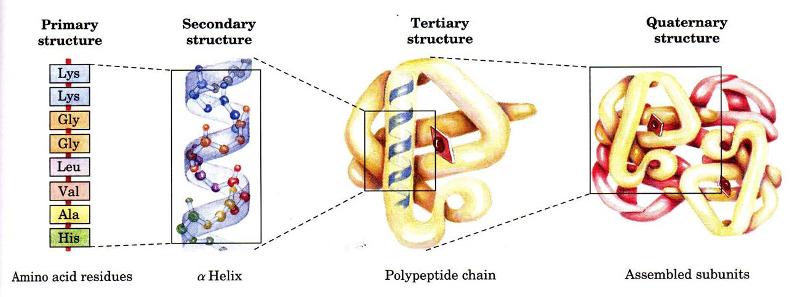
Define Denaturing of Proteins
Denaturation implies the destruction of the tertiary structure of a protein molecule and the formation of random polypeptide chains. The alteration of a protein's structure, leading to the loss of its biological function.
Define Enzymes
Globular proteins which act as catalysts(facilitate) of chemical reactions.
Define Active site
Region on the surface of an enzyme to which substrates bind and which catalyses a chemical reaction involving the substrates.
Define Immobilized Enzymes
Enzymes physically confined or localized in a certain defined region of space with retention of their catalytic activities, and which can be used repeatedly and continuously
Define DNA
Genetic component of an organism containing genetic code. A double helix made of two antiparallel strands of nucleotides linked by hydrogen bonding between complementary base pairs.
Define Nucleotides
Construction material of DNA. Consist of deoxyribose sugar, a phosphate group, and one of four nitrogenous bases (adenine, guanine, cytosine, or thymine)

Define Replication of DNA
The production of two DNA molecules from one original DNA molecule.
Define Transcription
The uses DNA as a template to make an RNA (mRNA) molecule. During transcription, a strand of mRNA is made that is complementary to a strand of DNA.
Define Translation
The process where messenger ribonucleic acid, or mRNA, synthesizes proteins. This is accomplished by the production of a chain of amino acids (a polypeptide chain) determined by the chemical information stored by a specific strand of mRNA. These polypeptides fold to form proteins.
Define Codons
A DNA or RNA sequence of three nucleotides that encoding a particular amino acid or signaling the termination of protein synthesis (stop codon).
Define Anticodon
Three nucleotide sequence located at one end of a transfer RNA (tRNA) molecule, which is complementary to a corresponding codon in a messenger RNA (mRNA) sequence.
Define Cell Respiration
The controlled release of energy from organic compounds to produce ATP.
Aerobic Respiration
Uses oxygen to produce energy
Anaerobic Respiration
Does not require oxygen and produces less energy
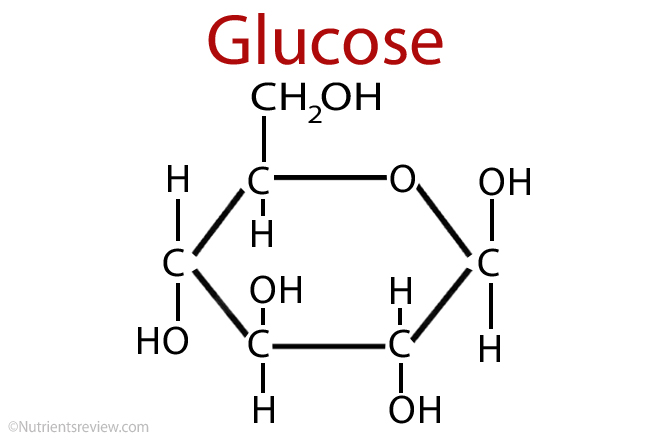
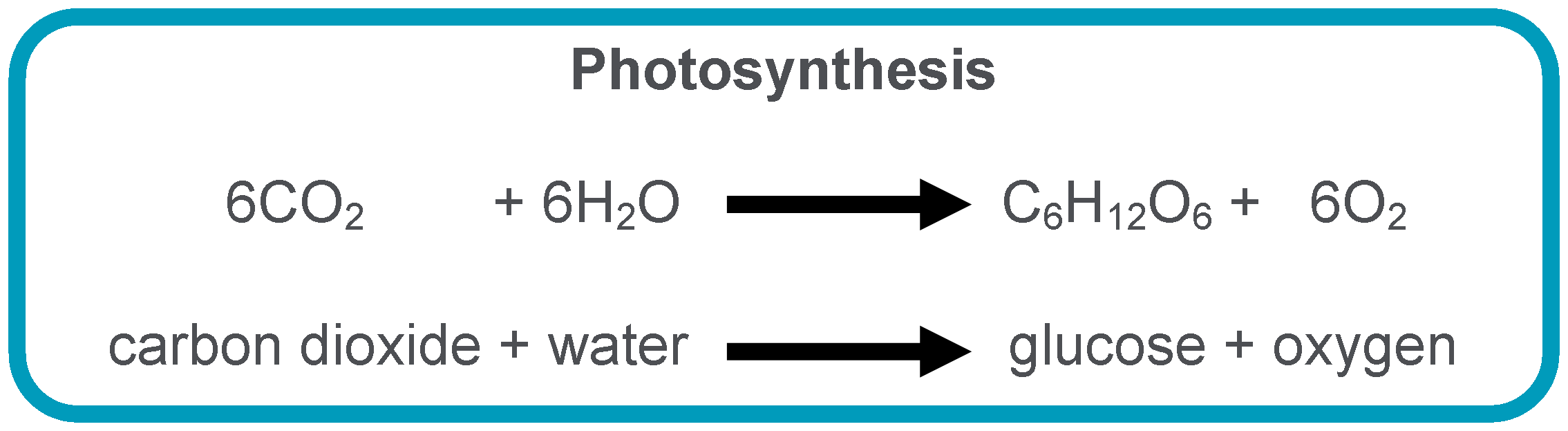
Define Photosynthesis
The process by which cells synthesise organic molecules (e.g. glucose) from inorganic molecules (CO2 and H2O) in the presence of sunlight. Light energy —> chemical energy
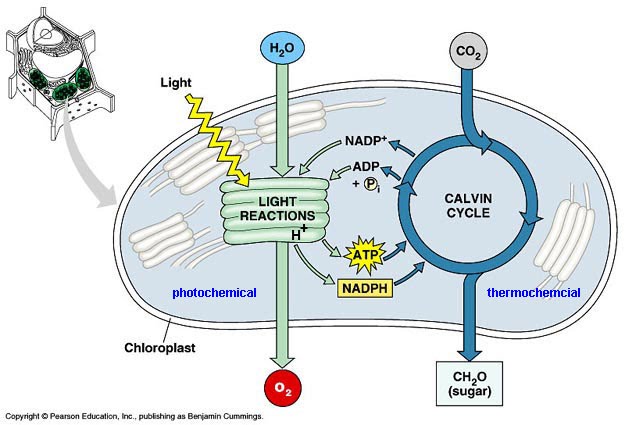
Define Limiting Factors of Photosynthesis
lacking of:
light
water
co2
chlorophyll
Water Molecule

One Water molecule having hydrogen bonds with 3 more water molecules

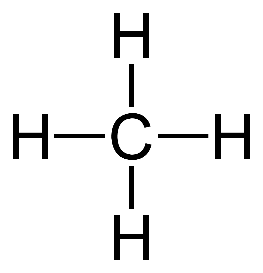
Methane
CH4
Amino Acid
R-CH(NH2)-COOH

Saturated fatty acid chain
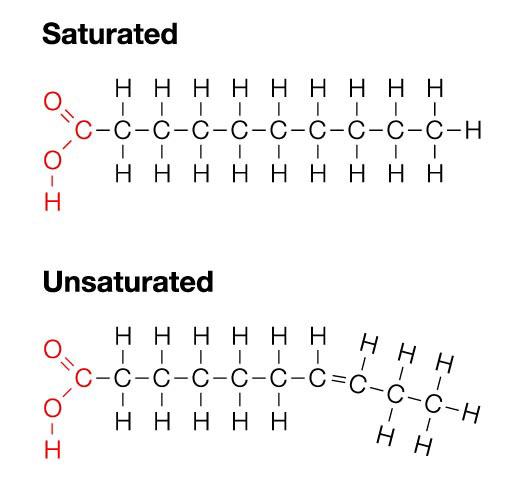
Unsaturated fatty acid chain
Trans - unhealthy (bad cholesterol) - unatural
Cis- healthy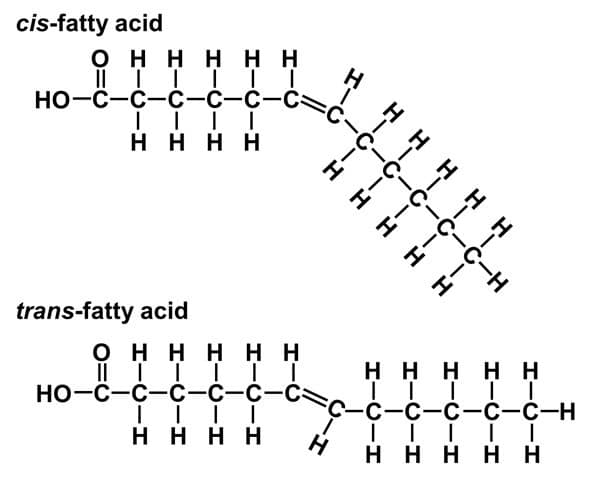
BMI
Weight (kg) / height² (m)

Chargaff’s Rule
Semiconservative replication of DNA - Meselson and Stahl’’s experiment
PCR
To amplify a sequence of DNA. Amplification is crucial for studies and diagnostics where only a small DNA sample may be available.
Components Required for PCR:
DNA Template: This contains the target region that needs to be amplified.
Primers: These are short, single-stranded DNA fragments that flank the target region and guide DNA polymerase to the correct location.
Taq Polymerase: Derived from the bacterium Thermus aquaticus, this enzyme is responsible for synthesizing new DNA strands.
Nucleotides (dNTPs): The building blocks (A, T, C, G) that the enzyme uses to build the new DNA strands.
Buffer and Magnesium Ions: These ensure the optimal chemical environment for the Taq polymerase to function.
Steps:
1. Denaturation
Temperature: 94–98 °C
Duration: 20–30 seconds
Purpose: Breaks the hydrogen bonds holding the DNA strands together, leading to two single strands.
2. Annealing
Temperature: 50–65 °C (dependent on primer sequence)
Duration: 20–40 seconds
Purpose: Primers bind (anneal) to their complementary sequences on the single-stranded DNA.
3. Extension
Temperature: 72 °C
Duration: 20–40 seconds per kb (kilobase)
Purpose: Taq polymerase adds nucleotides to the primers, extending the DNA strands.
4. Final Elongation
Temperature: 72 °C
Duration: 5–10 minutes
Purpose: Ensures that any remaining single-stranded DNA is fully extended.
5. Final Hold
Temperature: 4–15 °C
Purpose: Preserves the PCR reaction for subsequent analysis.
Applications of PCR
Genetic Disorders: Pinpoints specific mutations or genetic variations linked to diseases
DNA Fingerprinting: Compares DNA from crime scenes with samples from suspects.
Paternity Testing: Determines biological relationships between individuals.
Set up for the respirators

Condensation Reactions
The joining of two monomers. A hydrogen atom from one monomer and a hydroxyl group (OH) from another come together, forming a molecule of water (H₂O). This leads to the creation of a covalent bond between the two monomers.
Formation of disaccharides
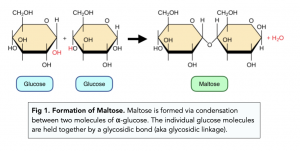 The covalent bond joining two monosaccharides together is a glycosidic bond
The covalent bond joining two monosaccharides together is a glycosidic bond
Maltose is formed via a condensation reaction between two molecules of α-glucose.
Sucrose is formed via a condensation reaction between a molecule of glucose and a molecule of fructose.
Lactose is formed via a condensation reaction between a molecule of glucose and molecule of galactose.
Formation of Dipeptide

Formation of Triglycerides

Hydrolysis
The splitting of monomers through the use of water.
Transport of Water
///
Solvent Properties of Water
Universal solvent - Polar nature can interrupt intramolecular forces and cause dissociation in other molecules.
Water as a coolant
Sweat is secreted by glands in the skin.
The high specific heat of vaporisation causes the water in sweat to take away a lot of heat from the tissues in the body when evaporating.
This helps cool the body during periods of intense activity.
Similarly, dogs pant and plants transpire.
Biological significance that are related to the properties of water
Significance of Water in Homeostasis
Homeostasis involves maintaining a stable internal environment, and water plays a central role in various aspects of homeostasis.
Water’s thermal properties help organisms maintain a stable internal body temperature, a critical aspect of homeostasis. For example, the high heat of vaporisation of water allows mammals to cool down via sweating.
Additionally, the solvent properties of water allow for the transportation of nutrients and waste materials in the body, which are crucial for maintaining homeostasis. For instance, water facilitates the transport of glucose, amino acids, and oxygen in the blood.
Comparing the thermal properties of water and methane
Water is made of two hydrogen molecules and one oxygen molecule, and due to the strong pull that electrons feel in the presence of oxygen, they tend to spend more time in the oxygen area than in the hydrogen area. This results in oxygen having a partial negative charge, and hydrogen having a partial positive charge. This phenomenon is known as polarity, and is part of what makes it so important to a living organism, as it can bond with other polar molecules and exhibit hydrogen bonding with other water molecules. Methane on the other hand, is made of one carbon atom and 4 hydrogen atoms. Like water, the bonds are covalent. However unlike water, carbon does not pull the electrons as strongly as oxygen does, and the balanced number of hydrogen atoms means that the pull carbon exerts on the electrons is cancelled out, making the molecule nonpolar overall. Because of its absence of polarity, the interaction between methane molecules is considered to be very weak, and can easily be broken. The following numbers are important, but it is still possible to gain points on the question without having memorised these numbers, as at this point you have explained sufficient difference in the two molecules. Because water displays hydrogen bonding with other molecules, a large amount of heat energy is required to break this, and water's boiling point is around 100 degrees celsius. Unlike water, methane's weak interactive forces results in a very low boiling point of around -161 degrees celsius. Their differing properties also affects the energy required for a phase change in both molecules. For water to change from liquid to gas (known as latent heat of vaporisation), it requires 40.7 degrees celsius due to its hydrogen bonding, but for methane to change from liquid to gas it only requires 8.2 degrees celsius; an important fact to note in its impact on the environment which we can talk about later.
Health Risks associated with fats
///
Functions of proteins with examples
Proteins are vital for the transport and storage of several molecules.
Haemoglobin: Found in red blood cells, this protein binds oxygen in the lungs and releases it in tissues where it's needed.
Ferritin: This protein's main function is to store iron in the liver, releasing it in a controlled fashion when needed.
Enzyme activity
Enzymes are specialised proteins that facilitate biological reactions, ensuring the cell's metabolic needs are met in an efficient manner.
They achieve this by offering an alternative reaction pathway with a reduced activation energy.
Substrate specificity: The intricate design of an enzyme’s active site ensures that only a particular substrate (or group of substrates) can bind, guaranteeing specificity.
Examples include digestive enzymes. Amylase breaks down carbohydrates in the mouth, while lipase, found in the pancreas, assists in fat digestion.
Factors that affect enzyme activity
Temperature (use water baths to minimise fluctuations)
pH (acidic or alkaline solutions)
Substrate concentration (choose range to avoid saturation)
Presence of inhibitor (type of inhibitor will be enzyme-specific)
Production of lactose free milk
Lactose is the sugar found in milk. It can be broken down by the enzyme lactase into glucose and galactose. However some people lack this enzyme and so cannot break down lactose leading to lactose intolerance. Lactose intolerant people need to drink milk that has been lactose reduced. Lactose-free milk can be made in two ways. The first involves adding the enzyme lactase to the milk so that the milk contains the enzyme. The second way involves immobilizing the enzyme on a surface or in beads of a porous material. The milk is then allowed to flow past the beads or surface with the immobilized lactase. This method avoids having lactase in the milk.
Replication of DNA with the roles of enzymes
A variety of enzymes are involved in the process of DNA replication. For replication to occur each strand of DNA must be separated from the other, a result of its double helix structure and the hydrogen bonds between complementary nitrogenous bases. The enzyme, DNA Helicase breaks the hydrogen bonds between the bases from the 5’ to 3’ direction, uncoiling the DNA and separating the strands. Thus, allowing other enzymes involved in the process to access each strand of DNA. Single strand binding proteins bind to each strand to ensure that the strands do not recoil and bind back together. The replication process then splits into two different processes occurring on the leading and lagging strands of DNA. A result of replication occurring in the 5’ to 3’ direction.On the leading strand RNA primase synthesises a short RNA primer that allows DNA polymerase III to bind to and begin adding DNA nucleotides in a 5’ to 3’ direction. Once this is finished, DNA ligase replaces this primer with DNA nucleotides. On the lagging strand, RNA primase places a few primers along the length of the strand. This allows DNA polymerase I to bind to these primers and add a few DNA nucleotides in the 5’ to 3’ direction. These short sequences of nucleotides are known as Okazaki fragments. The short gaps between the fragments are plugged up with nucleotides by DNA ligase and the primers are replaced with DNA nucleotides, forming a continuous strand.
Transcription of mRNA
Transcription: the synthesis of mRNA copied from the DNA base sequences by RNA polymerase, (the process by which an mRNA sequence is produced from a DNA template).
- RNA polymerase binds to a site on the DNA at the start of a gene
- RNA polymerase separates the DNA strands & synthesizes a complementary RNA copy from the antisense DNA strand
- Transcription occurs in a 5' to 3' direction: RNA polymerase adds the 5' end of the free RNA nucleotide to the 3' end of the growing mRNA molecule (in a 3' to 5' end in the antisense strand)
- Does so by covalently bonding ribonucleoside triphosphates that align opposite their exposed complementary partner (using energy from additional phosphate groups)
- Once RNA sequence synthesized; RNA polymerase will detach from the DNA molecule. RNA detaches from the DNA & double helix reforms.
- Transcription occurs in the nucleus (where DNA is) & once made mRNA moves to cytoplasm
Translation
Initiation
The first stage of translation involves the assembly of the three components that carry out the process (mRNA, tRNA, ribosome)
The small ribosomal subunit binds to the 5’-end of the mRNA and moves along it until it reaches the start codon (AUG)
Next, the appropriate tRNA molecule bind to the codon via its anticodon (according to complementary base pairing)
Finally, the large ribosomal subunit aligns itself to the tRNA molecule at the P site and forms a complex with the small subunit
Elongation
A second tRNA molecule pairs with the next codon in the ribosomal A site
The amino acid in the P site is covalently attached via a peptide bond (condensation reaction) to the amino acid in the A site
The tRNA in the P site is now deacylated (no amino acid), while the tRNA in the A site carries the peptide chain
Translocation
The ribosome moves along the mRNA strand by one codon position (in a 5’ → 3’ direction)
The deacylated tRNA moves into the E site and is released, while the tRNA carrying the peptide chain moves to the P site
Another tRNA molecules attaches to the next codon in the now unoccupied A site and the process is repeated
TerminationThe final stage of translation involves the disassembly of the components and the release of a polypeptide chain
Elongation and translocation continue in a repeating cycle until the ribosome reaches a stop codon
These codons do not recruit a tRNA molecule, but instead recruit a release factor that signals for translation to stop
The polypeptide is released and the ribosome disassembles back into its two independent subunits
Production of human insulin in bacteria
Insulin Production in Bacteria
Human Insulin Gene Isolation: Using restriction enzymes, the insulin gene is isolated from human DNA.
Insertion into Bacterial Plasmid: The insulin gene is inserted into a plasmid with specific markers.
Transformation into Bacteria: The recombinant plasmid is introduced into E. coli bacteria.
Culturing of Transformed Bacteria: The bacteria are cultured, leading to insulin production.
Insulin Extraction and Purification: Specific methods are used to extract and purify the insulin.
Advantages over Animal Insulin: Identical to human insulin, leading to fewer allergic reactions.
Respiration
organic compounds are broken down to release energy, which can be used in cells
source of organic compounds broken down is food
Carried out using enzymes carefully /controlled so that as much as possble is retained in a usable form
Adenosine triphosphate – form is a chemical substance (ATP)
Making ATP phosphate group is lined to ATP or ADP – energy to carry out the reaction comes from the breakdown of organic compounds
Aerobic respiration in humans
Aerobic respiration is the controlled released of energy in cells by converting organic molecules into ATP. It invovles the reduction and oxidation of electron carriers. Firstly the link reaction occurs where pyruvate from glycolysis is converted into acetyl coenzyme A, this is done by removing CO2 from a pyruvate and reducing a NAD molecule. Next in the Krebs cycle a four carbon molecule combines with the acetyl coenzyme A and a 6 carbon molecule is formed. Next the molecule is decarboxylated twice meaning two CO2 molecules are released and a 4 carbon compound remains. 3 reduced NAD molecules and a reduced FAD molecule are formed. There is some ATP also generated during the Krebs cycle. The four carbon molecule that remains will now start the next cycle with a new acetyl coenzyme A. Next the electron transport chain occurs, the now reduced NAD and FAD molecules carry the electrons to the inner membrane of the mitochondria. The electrons are deposited to carriers which enable proton pumping to occur, protons accumulate in the inter-membrane space and generate potential energy. Through diffusion the protons now pass through the ATP synthase enzyme embedded into the membrane and using their kinetic energy create ATP by combining an ADP molecule with an extra inorganic phosphate, this process is known as chemiosmosis. Finally as the electrons travel from carrier to carrier they reach the final terminal acceptor oxygen.
Anaerobic respiration in bacteria
Glycolysis
Overview of the Process
Glycolysis is the foundational stage of anaerobic respiration that occurs in the cytoplasm.
It breaks down one molecule of glucose into two molecules of pyruvate.
Consists of ten enzymatic reactions, divided into two main phases.
Energy Investment Phase
Steps 1 to 5: Consumption of 2 ATPs to modify glucose and split it into two 3-carbon molecules.
Phosphorylation: Glucose is phosphorylated twice, increasing its reactivity.
Splitting of Sugar: The resulting 6-carbon sugar diphosphate is split into two 3-carbon molecules.
Energy Payoff Phase
Step 6 to 10: Transformation of 3-carbon molecules into pyruvate, producing 4 ATP and 2 NADH.
Substrate-level Phosphorylation: Direct transfer of phosphate group to ADP.
Net ATP Yield: 2 ATP per glucose molecule, as 2 ATP are consumed and 4 ATP are produced.
Formation of Pyruvate
Pyruvate is the pivotal molecule formed at the end of glycolysis.
It can lead to different metabolic pathways depending on environmental conditions.
Photosynthesis
//
Absorption and action spectrum
Plants are green because they reflect the most green light. Plants are more efficient at absorbing red and blue light. An absorption spectrum shows all the light typically absorbed by a leaf. An action spectrum, meanwhile, shows all the light that's actually used for photosynthesis.
Effects of photosynthesis on earth
The process of photosynthesis happens in green plants where they take light, energy, water, and carbon dioxide and turn it into glucose and oxygen. This process is extremely important to life on Earth as it provides an energy source, food, and oxygen to living things; without this process, life would not exist.
Temperature, light intensity and CO2 concentration as limiting factors
Limiting factors in photosynthesis are conditions that directly affect the rate at which the process occurs. These include light intensity, carbon dioxide concentration, and temperature. Each factor plays a unique role and has a distinct impact on the photosynthetic rate.