Chemistry SAC 2: Rates and Equilibrium
1/47
Earn XP
Description and Tags
Chem SAC 2 Flashcards
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Rate of reaction
The change in concentration of reactants/products over time, measured in mol/L/s
Collision theory
For a reaction to occur, reactant particles must:
Collide with each other
Have sufficient energy to break reactant bonds
Collide in the correct orientation to break reactant bonds
Activation energy (Ea)
The minimum energy required to break reactant bonds in a collision.
High vs. low activation energy
High: strong reactant bonds, lower proportion of particles have the minimum energy required to react.
Low: weak reactant bonds, higher proportion of particles have the minimum energy required to react.
Transition state
A new arrangement of atoms when activation energy is absorbed - unstable because reactants break and products form
Reaction rate graphs
Graphs which measure amount of product/reactant vs. time
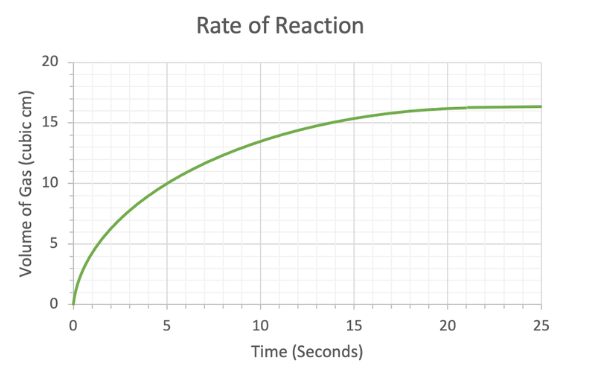
Reaction rate graph terms
Gradient: the rate of reaction
Tangent: the rate of reaction at any instant
Reaction rate conditions
Surface area of a solid reactant
Concentration of a dissolved reactant
Pressure of a gaseous reactant
Temperature
Presence of a catalyst
Concentration vs. reaction rate
Concentration: more dissolved reactant particles per unit volume
Increasing conc —> increasing frequency of collisions —> increasing successful collisions with correct orientation
Pressure vs. reaction rate
Pressure: adding more reactant gas/decreasing container volume
Increasing press —> increasing frequency of collisions —> increasing successful collisions with correct orientation
Surface area vs. reaction rate
Surface area: more particles are exposed at the surface when solids are broken down.
Increasing SA —> increasing frequency of collisions —> increasing successful collisions with correct orientation
Temperature vs. reaction rate
Temperature increases energy of collisions —> increased collisions with >= activation energy —> increases successful collisions
Temperature causes faster-moving particles —> increases frequency of collisions —> increases successful collisions
Energy increase has a greater effect than frequency of collisions on reaction rate
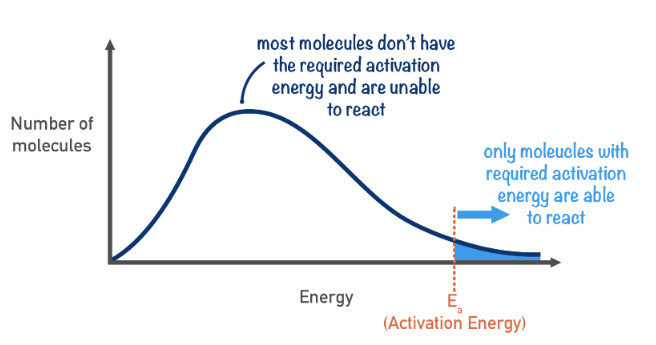
Maxwell-Boltzman distribution
A graph that shows the kinetic energy range of particles in a substance
Higher temperatures lead to an increase in an energy, hence a much greater proportion of particles have >= activation energy
Homogenous catalyst
Catalysts in the same physical state as the reactants & products
Heterogenous catalyst
Catalysts in a different physical state as the reactants & products
More easily separated from products
Reused easily
Often used at high temps
Catalysts vs. reaction rate
Greater proportion of reactant collisions >= activation energy —> leads to a chemical change
Irreversible chemical reaction
A reaction that proceeds in one direction, indicated by one-way arrows
Reversible chemical reaction
A reaction that goes in two directions, where products can reform reactants.
Open system
A system where matter and heat energy can be exchanged with the surroundings.
Closed system
A system where matter cannot be exchanged with the surroundings, but heat energy can.
Equilibrium
The rates of both forward and reverse reactions are equal
Concentrations and amounts are constant
Temperature is constant
Mixture of both reactants and products
Dynamic equilibrium
Both forward and reverse reactions are occurring at the same rate —> bonds constantly break/reform
Homogenous equilibria
An equilibrium system that has all reactants and products in the same state
Heterogenous equilibria
An equilibrium system that has reactants and products in different states
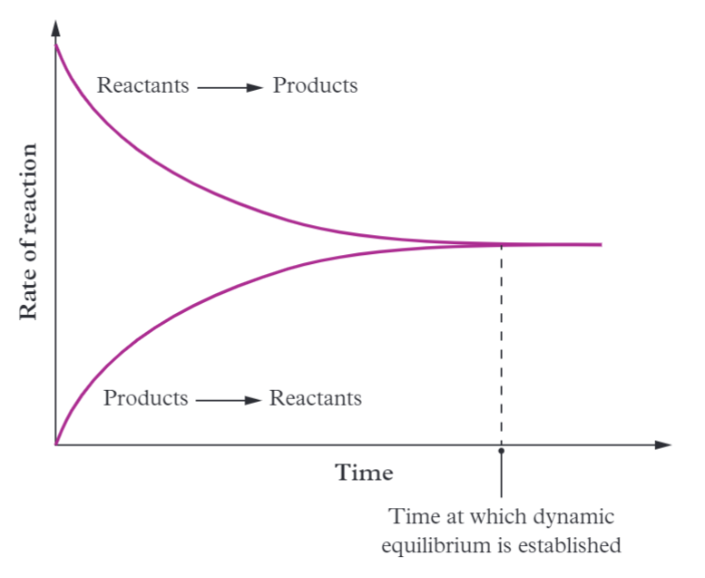
Rate-time graph
A graph where equilibrium is reached and when the forward & reverse reaction rate is the same.
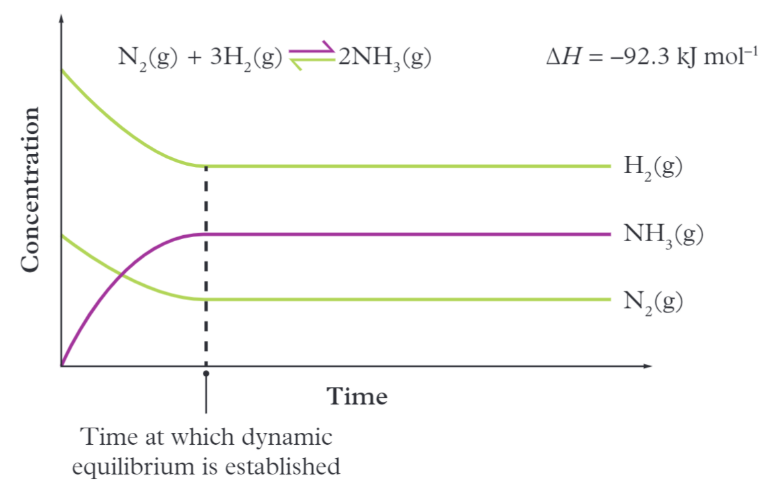
Concentration-time graph
A graph where equilibrium is reached and when the concentration of all species is constant
Extent of reaction
The relative amount of reactants and products present at a particular time, and does not give information on how fast the reaction proceeds.
Reaction quotient (Q)
A numerical measure of the extent of a reaction
For the equation aW + bX <=> cY + dZ, Q is calculated by:
([Y]^c * [Z]^d) / ([W]^a + [X]^b)
Equilibrium constant (K)
The reaction quotient (Q) at equilibrium (fixed temp)
For the equation aW + bX <=> cY + dZ, K is calculated by:
([Y]^c * [Z]^d) / ([W]^a + [X]^b)
Units for equilibrium constants & reaction quotients
Use M (mol/L) for units:
For the example [NH3]² / [H2]³ * [N2], the units should be M^-2 (from 2 - (3 + 1) through index laws)
Q & K: relative reactants and products
Q/K < 10^-4: value of Q/K is small which suggests that there are mostly reactants in a mixture
10^-4 < Q/K < 10^4: value of Q/K is moderate which suggests that there are a significant amount of both reactants and products.
Q/K > 10^4: value of Q/K is large which suggests that there are mostly products in a mixture
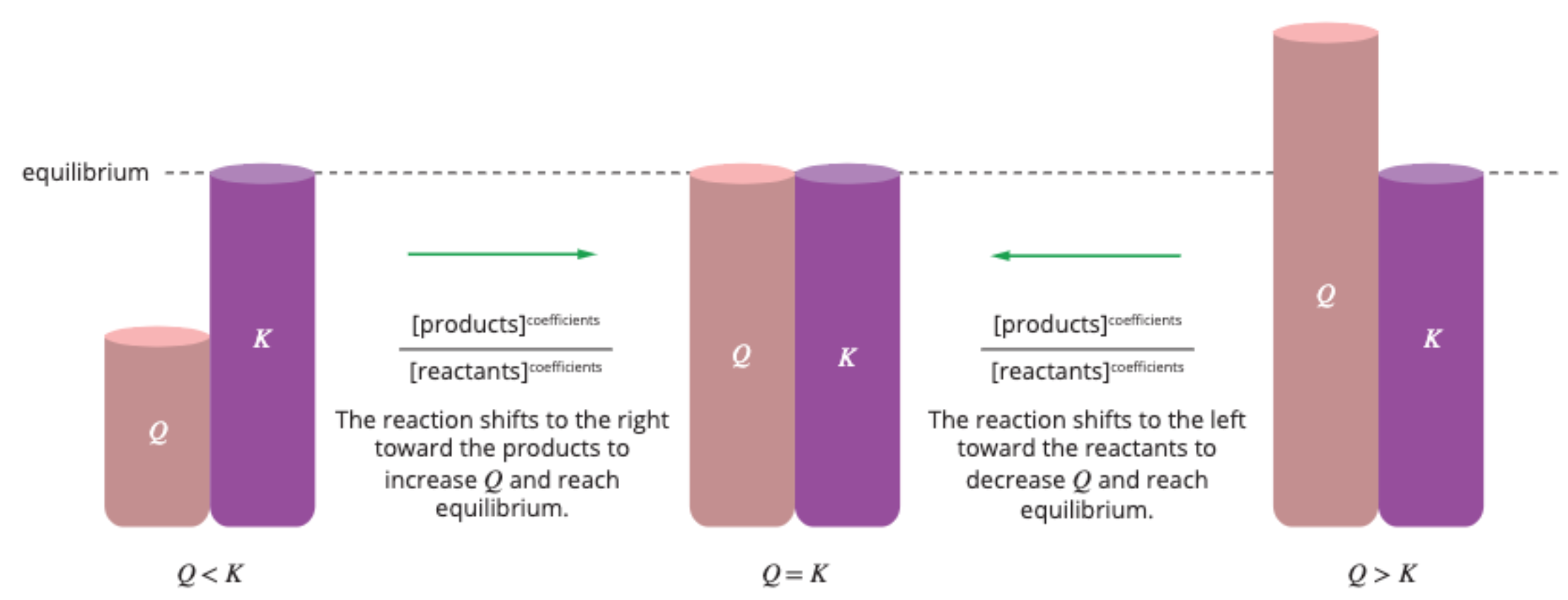
Q vs. K
Q > K: the reaction shifts to the ‘left’ and favours the reverse reaction to reach equilibrium (by forming more reactants)
Q < K: the reaction shifts to the ‘right’ and favours the forward reaction to reach equilibrium (by forming more products)
Q = K: the reaction is at equilibrium and does not shift.
K in different equations
if one reaction is the reverse of another, the two equilibrium constant (K) values are reciprocal of each other
If the coefficients of a reaction are doubled, the value of K is squared.
If the coefficients of a reaction are halved, the value of K is square rooted.
RICEC tables
R = Reaction, I = Initial, C = Change, E = Equilibrium, C = Concentration
1. R: Write the reaction down
2. I: Identify the initial mole amounts of reactants and products (0)
3. C: Calculate the change in the reactants (-) and products (+) according to the mole ratio
4. E: Minus the I and C row to calculate the amounts at equilibrium
5. C: Calculate the concentrations using n/V and hence, find K (equilibrium constant)
Position of equilibrium
The relative amounts of reactants and products at equilibrium. It is affected by:
Adding/removing reactants/products
Changing pressure by changing volume
Dilution (aqueous equilibria)
Changing temperature
Le Chatelier’s principle (LCP)
If a system at equilibrium is subjected to a change, a net reaction will occur that partially opposes the change and the system will establish a new equilibrium.
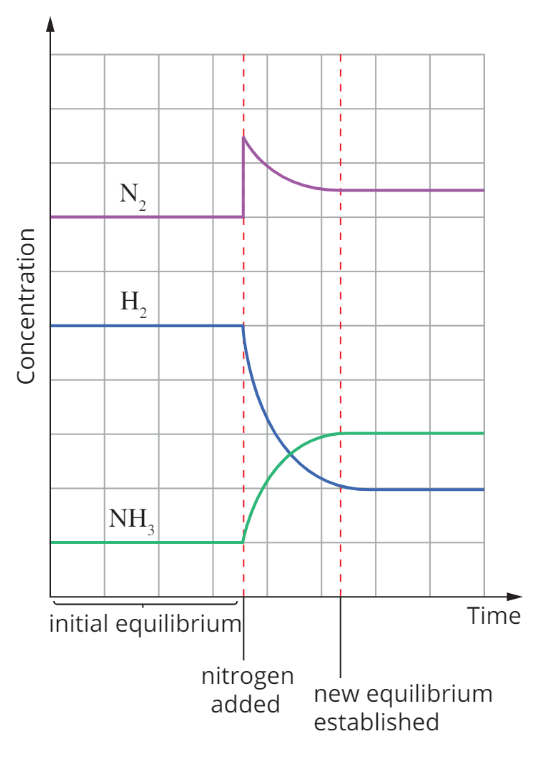
Adding reactants/removing products
The reaction shifts to the ‘right’ and favours the forward reaction to consume reactants/increase products
Eg. N2 + 3H2 —> 2NH3: the new concentration of N2 is still higher than its original concentration, the change was partially opposed.
K does not change with concentration
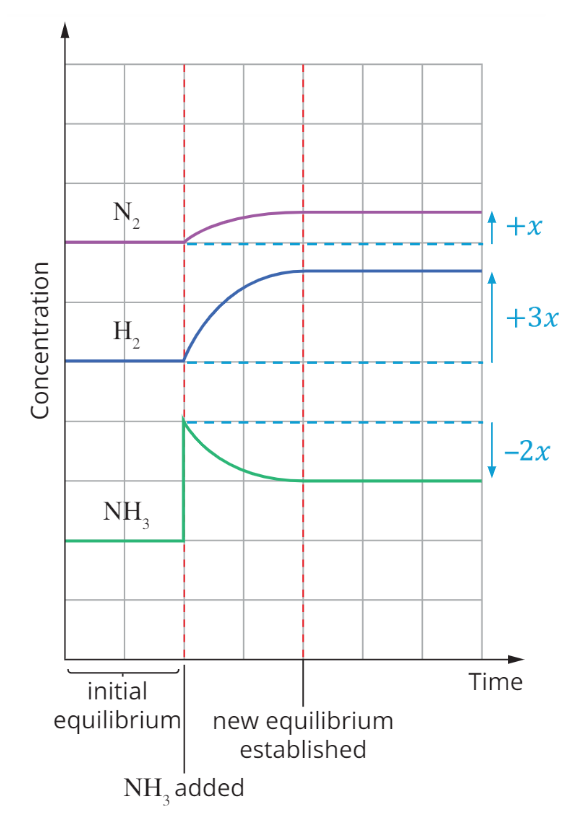
Adding products/removing reactants
The reaction shifts to the ‘left’ and favours the reverse reaction to partially oppose the change (decrease products/increase reactants)
Shifts in concentration follow the mole ratio in the balanced equation.
K does not change with concentration
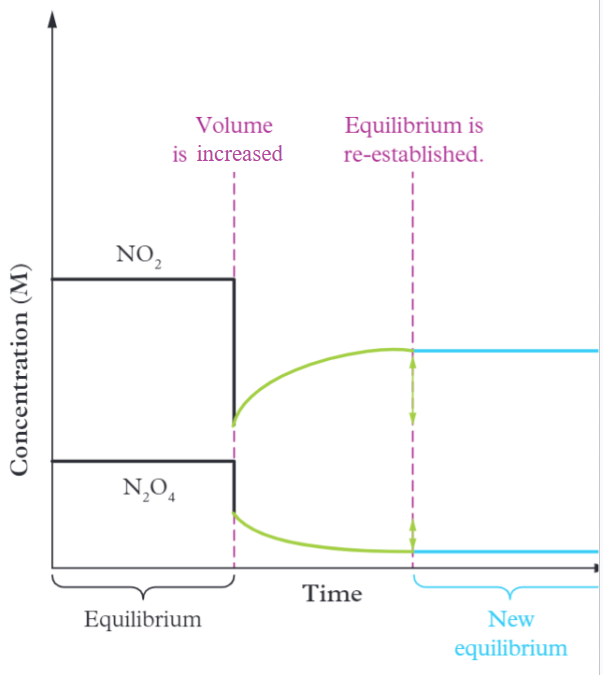
Increasing volume/decreasing pressure
The reaction shifts to the side with more particles and concentrations drop at the new equilibrium
Decreasing volume/increasing pressure
The reaction shifts to the side with less particles and concentrations remain higher at the new equilibrium
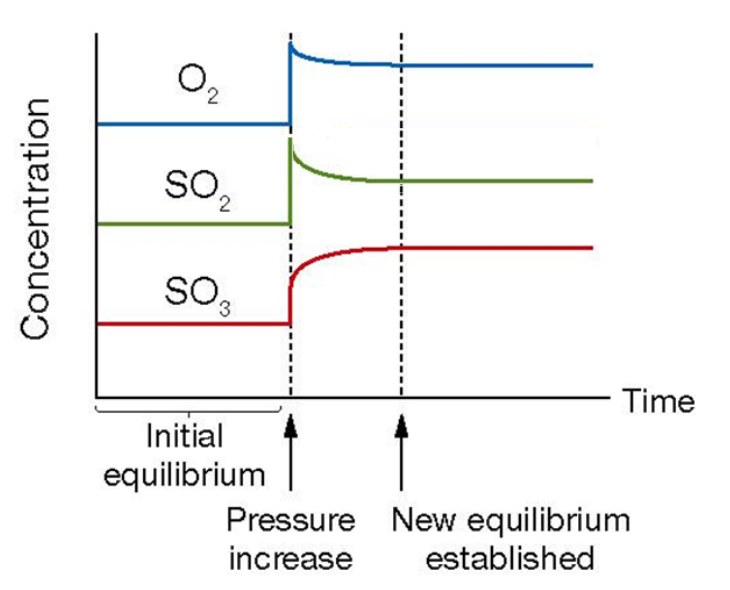
Volume changes using equilibrium law
If volume is halved, then concentration will double (c = n/v), leading to K > Q, hence a net forward reaction must occur to partially oppose the change.
Changing pressure by adding inert gases
The addition of an inert gas (eg. He) will increase overall pressure in a gaseous equilibrium
The concentrations of the reactants remain the same, so there is no change in equilibrium position
Dilution (adding water)
Adding water reduces the number of dissolved particles per unit volume
Hence the reaction shifts to the side with more dissolved particles
Eg. Fe3+ + SCN- —> FeSCN2+ dilution would result in a reverse reaction as the reactants have 2 particles compared to 1.
Temperature increase vs. K
Temperature increase means that heat energy is added to a reaction, and the system must consume heat energy to partially oppose the change.
Endothermic: shifts right, more products, K increases
Exothermic: shifts left, more reactants, K decreases
Temperature decrease vs. K
Temperature decrease means the system moves in the direction that releases heat energy
Endothermic: shifts left, more reactants, K decreases
Exothermic: shifts right, more products, K increases
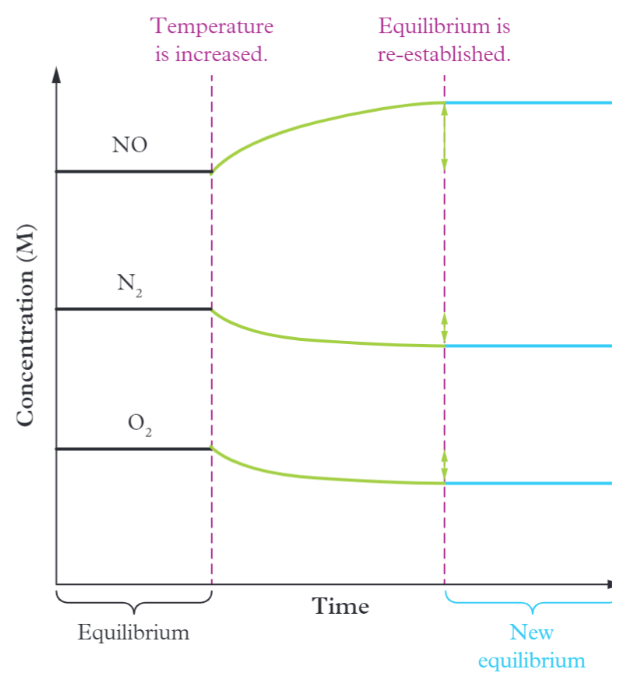
Example of changing temperature
Eg. N2 + O2 —> 2NO deltaH = +180kJ
A temp increase will shift the reaction to the right and K increases
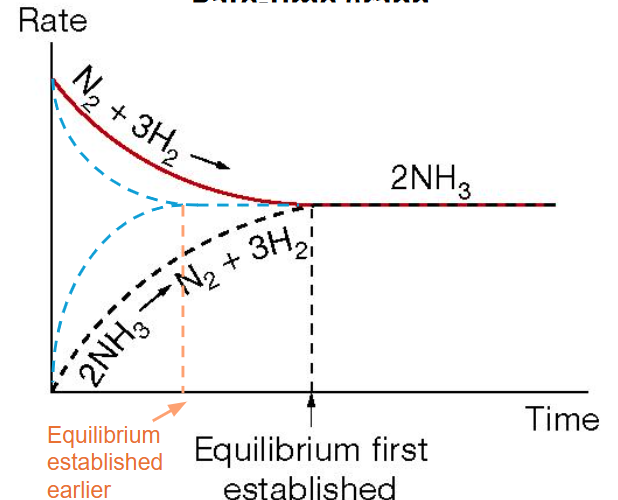
Catalysts
Catalysts provide an alternate reaction pathway by lowering activation energy, hence a greater proportion of particles have the energy needed for successful collisions.
Increases both forward & reverse reactions
Do not change equilibrium position or K
Increase the rate at which equilibrium is established.
Yield
The amount of desired product from a chemical reaction.