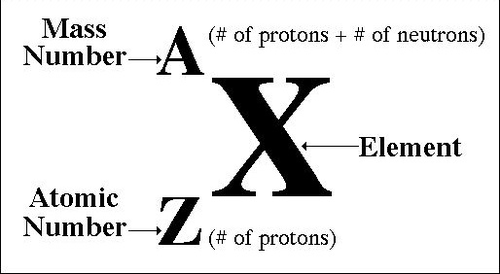Chemistry Unit 2.1: Atomic Structure
5.0(1)
Card Sorting
1/71
Earn XP
Description and Tags
Atomic Structure notes from all google slides.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
1
New cards
Democritus
Was the first person to think about an atom's existence.
2
New cards
Democritus believed
matter is composed of atoms, tiny indivisible particles
3
New cards
Democritus didn't have
experimental evidence to support his thoughts.
4
New cards
Aristotle was
A very famous and popular Greek philosopher who rejected the idea of atoms
5
New cards
Aristotle was so influential that
he was able to have Democritus' ideas rejected
6
New cards
But Aristotle and Democritus both agreed that
matter is made of basic components.
7
New cards
John Dalton
developed modern atomic theory
8
New cards
Dalton's Atomic Theory 1st Point
All elements are composed of indivisible particles called atoms.
9
New cards
Dalton's Atomic Theory 2nd Point
Atoms of the same element are identical. The atoms of any one element are different from those of another. (This is partially incorrect, as Isotopes exist.)
10
New cards
Dalton's Atomic Theory 3rd Point
Atoms of different elements mix or combine in whole number ratios.
11
New cards
Dalton's Atomic Theory 4th Point
Chemical reactions occur when atoms separate, join, or rearrange.
12
New cards
In a chemical reaction, atoms of one element NEVER
change into another.
13
New cards
Cathode Ray Tube Experiment was made by J.J Johnson. In the experiment there was
A tube with inert gas, and two plates, a positive and a negative.
14
New cards
The particles in the gas were attracted to the
positive plate.
15
New cards
Therefore, the particles MUST
have a negative charge.
16
New cards
J.J. Thomson
Discovered the electron
17
New cards
J.J. Thomson had believed that the atom was ____________________________________________________________ from his experimental evidence.
a solid positive sphere with electrons shoved into the sides of it.
18
New cards
JJ Thomson Model was named
Plum Pudding Model
19
New cards
Robert Millikan
used the oil-drop apparatus to determine the charge of an electron
20
New cards
The mass of an electron is at least
1000 times smaller than the lightest atom. The mass of the electron is negligible.
21
New cards
Ernest Rutherford
Gold foil experiment
22
New cards
Ernest Rutherford used the gold foil experiment to
discover the nucleus.
23
New cards
Ernest Rutherford had shot high energy beam of
alpha particles into gold foil.
24
New cards
Most of the alpha particles went through he concluded...
The atom is mostly empty space.
25
New cards
Few particles were deflected at small angles he concluded...
The alpha particle came close to something small and positive (nucleus).
26
New cards
Very rarely particles were deflected at large angles he concluded...
The alpha particles hit a small, very dense, and positively charged center (nucleus).
27
New cards
Niels Bohr's Atom
electrons orbit in energy levels around the protons in the nucleus like our solar system.
28
New cards
Erwin Schrödinger
Electron cloud model: doesn't stay in fixed orbits, rather stay in a three dimensional space.
29
New cards
Electrons do not follow
fixed orbits.
30
New cards
Electron Bohr vs Electron Cloud
Electron Bohr stays in fixed orbits like the the solar system, it doesn't diverge from it's path. While an electron cloud is a three dimensional region, the energy levels still exist, but they don't orbit in fixated states.
31
New cards
Werner Heisenberg
Developed an uncertainty principle
32
New cards
uncertainty principle
we cannot determine the speed and position of the electrons around the nucleus.
33
New cards
quantum mechanics theory
electrons are found in specific orbitals
34
New cards
James Chadwick
discovered the neutron.
35
New cards
neutron
no charge
36
New cards
Atomic Number
the number of protons in the nucleus of an atom
37
New cards
An atomic number is always
a whole number
38
New cards
number of protons is equal to
number of electrons
39
New cards
the number of protons
will never change
40
New cards
Atomic Mass
The average mass of all the isotopes of an element
41
New cards
An Atomic Mass
will have a decmial
42
New cards
Atomic Number Vs Atomic Mass
Atomic Number is above the element, the mass is below.
43
New cards
Mass Number
Number of protons and neutrons combined
44
New cards
The atomic mass number is always
a whole number
45
New cards
Neutrons =
mass number - atomic number
46
New cards
The oxygen mass number is 16 and its atomic number is 8, what is the number of neutrons
8
47
New cards
Isotopes
Atoms of the same element that have different numbers of neutrons
48
New cards
Isotopes are
chemically alike
49
New cards
Isotopes have
different mass numbers
50
New cards
Hyphen Notation
Element-mass number
51
New cards
Isotopic Notation
A symbol that identifies the isotope of an element
52
New cards
Atomic Mass
The average mass of all the isotopes of an element
53
New cards
Atomic mass formula
Convert percentage back to decimal,
54
New cards
Multiply decimal with mass number
55
New cards
Add products of each isotope together
56
New cards
Chlorine has two common isotopes, with the mass of 35.45 amu (75.00% abundance) and 37.29 amu (25.00% abundance) What's the Atomic Mass?
35.91
57
New cards
What if you do not have the percentage or the atomic mass
Calculate the atomic mass of one of indium's isotopes (there are two)
58
New cards
If the atomic mass of indium is 114.8200 amu and one isotope of indium has a mass of 114.9041 amu and an abundance of 95.795%, whats the atomic mass?
113 amu
59
New cards
Atomic #
# of protons = # of electrons (neutral atoms)
60
New cards
Mass #
protons + neutrons
61
New cards
Neutrons
mass # - atomic #
62
New cards
Ion
An atom or group of atoms that has a positive or negative charge.
63
New cards
How do ions form
When an atom gain or lose electrons
64
New cards
How to write an ionic charge
An ionic charge is written as a sign (positive '+' or negative '-') followed by a number.
65
New cards
What does the number represent in an ion
the amount of electrons gained or lost
66
New cards
cation
A positively charged ion
67
New cards
metals lose electrons to become
cations
68
New cards
Cations have the same name as the element that forms them but
different chemical properties
69
New cards
Anions
negatively charged ions
70
New cards
Nonmentals tend to form ions
by gaining electrons
71
New cards
The name of the anion is the element name
with "ide" on the end
72
New cards
Ions Abbreviations