LC CHEMISTRY- EXPERIMENT: TO MEASURE THE RELATIVE MOLECULAR MASS OF A VOLATILE LIQUIDLC CHEMISTRY- EXPERIMENT: TO MEASURE THE RELATIVE MOLECULAR MASS OF A VOLATILE LIQUID
1/14
Earn XP
Description and Tags
REBECCA'S LC CHEMISTRY- TO MEASURE THE RELATIVE MOLECULAR MASS OF A VOLATILE LIQUID KNOWT
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Volatile liquid
liquid with low boiling point
Relative molecular mass
average mass of a molecule compared to 1/12 of the carbon 12 isotope
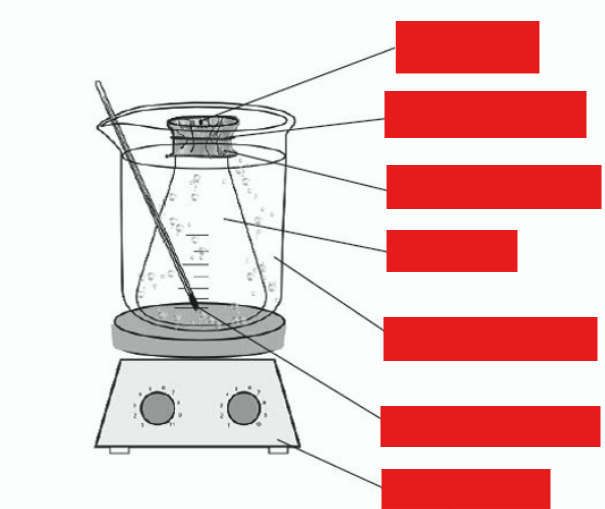
label this diagram
pinhole
aluminium foil
boiling water
vapour
hotplate
rubber band
thermometer
How is mass of volatile liquid measured (using words, 3 points needed)
get mass of flask and foil intially
heat until liquid is gone
get mass of flask and foil at the end by subtraction the initial and final value.
How is volume of container measured (using words, 2 points needed)
fill flask with water
empty into graduated cylinder
How temperature at which temperature took place
use thermometer to measure temperature of water
How could the pressure be measured
barometer
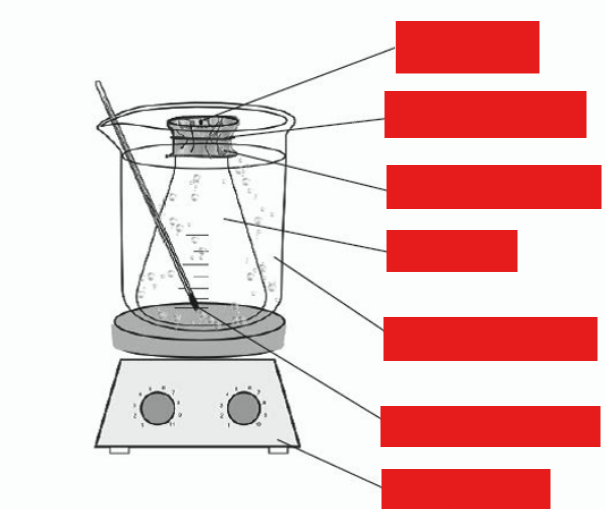
describe this diagram using words with diagram
flask covered with foil with pinhole
immersed so that at least half is underwater
how is the volatile liquid vapourised
hotplate under beaker
why pressure of vapour at the end of heating stage of the experiment was known to be equal to the atmospheric pressure
vapour escaped due to pinhole
describe HOW to get that mass of the vapour in the container at the end of the heating stage was found
subtract mass of flask initially from mass of flask at end
account for volatility of bromine boiling point 58.8c compared to that of water 100 c
hydrogen bonds in water
example of volatile liquid suitable for this experiment
acetone (propanone)
why is the method unsuitable for liquids that are non-volatile/unstable
high boiling point
What modern instrumental technique could be used as a more accurate method to measure the relative molecular masses of volatile and non-volatile liquids as well as of solid and gaseous substances?
mass spectrometer