How do concentration of a reactant and temperature affect the rate of a chemical reaction
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
Outline the experiment
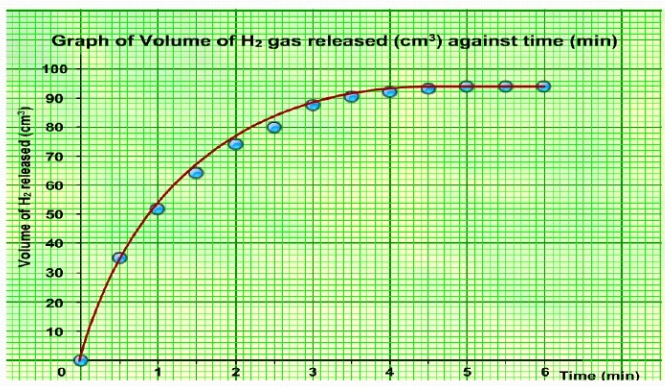
Investigate the rate of reaction between magnesium and hydrochloric acid
measure the volume of hydrogen gas produced
Effect of concentration on rate
Fill a trough with water and invert a measuring cylinder full of water into it.
Add a fixed length of magnesium ribbon to a conical flask.
Add HCl of known concentration (e.g. 1.0 mol/dm³).
Quickly seal with a bung and delivery tube connected to the measuring cylinder.
Start a stopwatch and record volume of hydrogen gas every 10 seconds.
Repeat with different concentrations (e.g. 0.5, 1.0, 1.5 mol/dm³).
Effect of temperature on rate
Warm HCl to a set temperature using a water bath (e.g. 30°C).
Fill a trough with water and invert a measuring cylinder full of water into it.
Add a fixed length of magnesium ribbon to a conical flask.
Add the heated HCl, seal quickly with bung and delivery tube.
Measure volume of hydrogen gas every 10 seconds.
Repeat at different temperatures (e.g. 30°C, 40°C, 50°C), keeping concentration constant.
What were the findings
Higher concentration = more particles = faster rate of reaction (more collisions per second).
Higher temperature = particles move faster = more frequent and energetic collisions → faster rate.
Plotting the graph
x - axis: volume of gas
y - axis: time