9: Alkanes
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
59 Terms
hydrocarbon structure
each carbon atom in an alkane is surrounded by 4 bps of electrons so the shape around each carbon atom is a tetrahedral and the bond angles are 109.5 degrees
polarity, structure, bonding
• carbon and hydrogen have similar electronegativity so the bonds are nonpolar
• this means that all alkane molecules will also be nonpolar
• alkanes have a simple molecular structure with van der Waals. these are weak intermolecular forces
solubility
The forces of attraction between water molecules are hydrogen bonds which are much stronger than the van der Waals in alkanes, therefore alkanes are not soluble in water
trend of boiling points in straight chain alkanes
As the length of the carbon chain increases, the boiling point of the alkane increases
explanation
this is because there are more electrons in the molecule so the van der Waals forces between the molecules become stronger and require more energy to break
branched chain alkenes trend
no. branches increases = bp decreases
explanation - brancehd bp
this is because there are fewer points of contact between the molecules so the van der Waals forces between molecules become weaker and require less energy to break
crude oil is a mixture of
mainly alkane hydrocarbons
fractional distillation
The separation of the components of a liquid into fractions which differ in boiling point
step 1
1. crude oil is vaporized and vapour is introduced near the bottom of the column
step two
The vapour rises up the column and creates a temperature gradient
step three
because the alkanes have different boiling points they condense at different levels and the frictions are collected
step 4
the hydrocarbons with the lowest boiling points do not condense and I drawn off as gases at the top of the tower
step 5
The largest hydrocarbons do not vaporize at all and are collected at the base of the tower as a thick residue
order of fractions
20°: petroleum gas
150°: gasoline (petrol)
200°: kerosene
300°: diesel
370°: industrial fuel oil
400°: lubricating oil, paraffin wax and bitumen
Why are hydrocarbons cracked
longer, less useful alkanes are converted to more useful shorter molecules in which CC bonds are broken. The demand for petrol, diesel and jet fuel does not match the natural abundancies in a barrel of crude oil
two types of cracking
thermal and catalytic
thermal cracking temperature and pressure
very high temperatures and very high pressure
products - cracking thermal
alkanes and high percentage of alkenes
Why - thermal cracking products
The CC bonds can break at different positions in the chain to give a mixture of products.
What are the products used for
to make polymers
catalytic cracking temperature and pressure
high temperature and a slight pressure
conditions
in the presence of a zeolite catalyst
products
cycloalkanes, branched alkanes, aromatic hydrocarbons such as benzene
What r products used for
used as motor fuels
complete combustion products
co2, h2o
equation
CnH2n+2 + O2 --> CO2 + H2O
incomplete combustion products
co, h2o
equation
CnH2n+2 + O2 --> CO + H2O
when does incomplete combustion occur
when there is a limited supply of o2
further incomplete combustion products
solid C (soot), h2o
equation
CnH2n+2 + O2 --> C + h2o
when does this occur
when there is a very limited supply of oxygen
pollutants from combustion
unburned hydrocarbons, carbon dioxide, carbon monoxide, carbon, nitrogen oxides, sulphur dioxide
unburned hydrocarbons effect + production
effect: low level ozone (causes respiratory problems)
production: reacts with NOx gas to form low level ozone
carbon dioxide effect + production
effect: global warming
production: complete combustion of fuels
carbon monoxide effect + production
effect: toxic gas
production: incomplete combustion of fuels in limited supply of oxygen
carbon effect + production
effect: particles exacerbate asthma
production: further incomplete combustion in very limited supply of oxygen
nitrogen oxides effect + production
effect: acid rain and photochemical smog
production: N2 + O2 from the air react at high temperatures in engine, e.g N2 + O2 --> 2NO
sulphur dioxide effect + production
effect: acid rain
production: s from fuel impurities reacts with O2 in air, S + O2 --> SO2
What do catalytic converters remove
CO, NO and unburned hydrocarbons
structure catalytic converters
contain honeycombed structure with a thin layer of Pt/Pd/Rh metals
Why is a thin layer of metals used
to reduce the amount needed - to reduce the cost
Why is a honeycomb structure used
a large surface area
removal of NO + CO, + equation
NO + CO react to produce less polluting products
2NO + 2CO --> 2CO2 + N2
removal of unburnt hydrocarbons 1
by reacting with o2
C8H18 + 12.5O2 --> 8CO2 + 9H2O
removal of unburnt hydrocarbons 2
by reacting with NO
C8H18 + 25NO --> 8CO2 + 9H2O + 12.5N2
flue gas desulfurization
when power stations burn coal or natural gas to produce electricity sulphur dioxide is also produced
What are chimneys coated with which absorb and react with sulphur dioxide produced. include equations.
calcium oxide or calcium carbonate.
SO2 + CaO --> CaSO3
SO2 + CaCO3 --> CaSO3 + CO2
alkanes s are generally unreactive because
CC and CH bonds are strong
alkanes are nonpolar
do halogens react with alkanes to form halogenoalkanes
yes they do
reagent + conditions
halogen eg Cl2 or Br2 ok
UV light
type of reaction
substitution because the hydrogen atom is replaced by a halogen atom
ethane + chlorine formula equation
H H H H
| | | |
H-C-C-H + Cl2 --> H-C-C-Cl + HCl
| | | |
H H H H
how were position isomers of halogen alkenes formed
when three or more Cs react with a halogen
further substitution
if an alkane is reacted with an excess halogen each hydrogen atom can be replaced in turn by a halogen atom
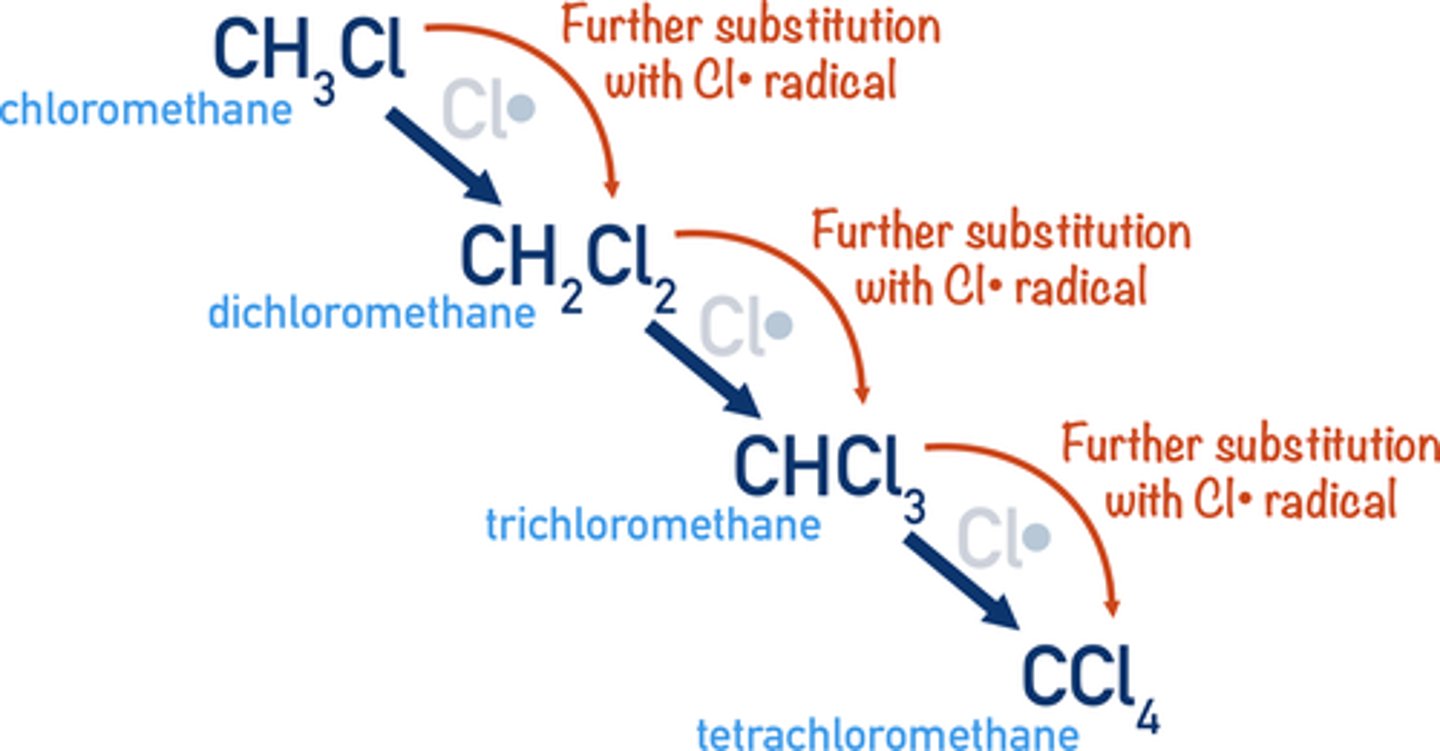
radical
a species with an unpaired electron
the 3 stages of free radical substitution
initiation: formation of radicals
propagation: formation of products
termination: removal of radicals
initiation, propagation and termination for:
CH4 + Cl2 --> Ch3Cl + HCl
1. initiation: Cl2 --> 2Cl•
2. propogation:
CH4 + Cl• --> •CH3 + HCl (•CH3 is an intermediate)
•CH3 + Cl2 --> CH3Cl + Cl• (Cl• is a catalyst)
3. termination:
•CH3 + Cl• --> CH3Cl
Cl• + Cl• --> 2Cl
•CH3 + •CH3 --> C2H6