Bio 98: Ch. 2 - Water: The Chemistry of Life
1/5
Earn XP
Description and Tags
2.1 Weak Interactions in Aqueous Systems / 2.2 Ionization of Water, Weak Acids, and Weak Bases / 2.3 Buffering Against pH Changes in Biological Systems
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
Which statement about water is correct? (SC2.1)
b. The O atom in H2O has a partial negative charge.
Which atoms can participate in H bonds? (SC2.2)
Nitrogen (electronegativity = 3.04)
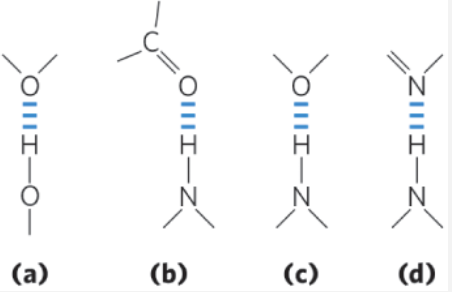
A carbonyl group is the H-bond acceptor in which molecule? (SC2.3)
(b)
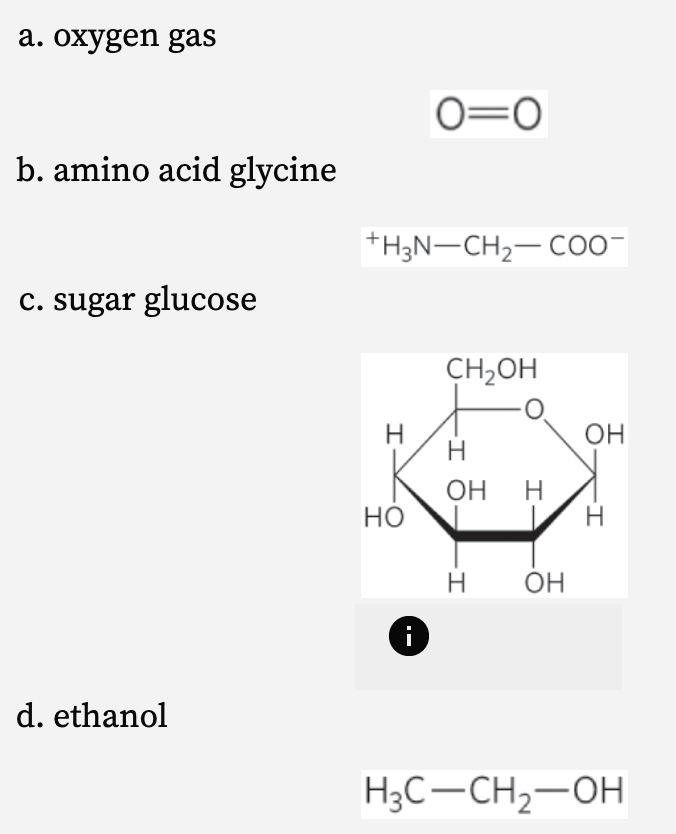
Which substance is LEAST soluble in water? (SC2.4)
oxygen gas
Which statement is true of the hydrophobic effect? (SC2.5)
Association of nonpolar molecules in water is facilitated by an increase in the overall entropy of the system.
Of the weak interactions and effects discussed in this section, which confers the largest net gain in stability in large macromolecules?
the hydrophobic effect