chemistry module 6 carbonyls
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
what are two types of carbonyls
aldehydes and ketones
what is the most common reaction for carbonyls to undergo
nucleophilic addition
process of nucleophilic addition
the carbonyl group in aldehydes and ketones is polarised. the oxygen atom is more electronegative than carbon, drawing electron density towards itself. this leaves the carbon slightly positive and the oxygen slightly negative. the carbon compound is therefore susceptible to attack by a nucleophile.
what is the general mechanism for nucleophilic substitution for aldehydes

what is the general mechanism for nucleophilic substitution for ketones

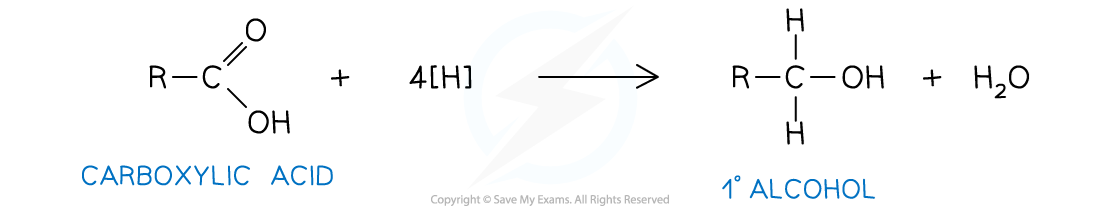
equation for carboxylic acid to a primary alcohol:
equation for aldehyde to a primary alcohol:

equation for ketone to a secondary alcohol:
